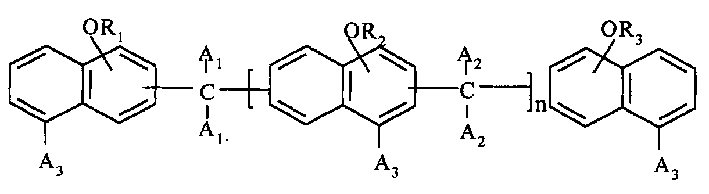
Polyfunctional epoxy resin and its preparation method
A base epoxy resin and multifunctional technology, which is applied in the field of multifunctional epoxy resin and its preparation, can solve the problems of large addition amount, increased resin viscosity, poor product stability in use, etc., and achieves low water absorption, high Effects of heat resistance, excellent heat resistance and dimensional stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 288g of 1-naphthol, 30g of paraformaldehyde and 800mL of toluene into a reaction flask equipped with a thermometer, constant flow pump, condenser and stirrer, heat to 70°C, and slowly add 4.2g of citric acid. After the addition, the temperature was raised to 100° C., and the reaction was refluxed for 8 hours. After the reaction was completed, neutralize the reaction mixture with 10% aqueous sodium carbonate solution, wash to neutrality with distilled water, and remove solvent, water and unreacted monomers in the organic phase under reduced pressure to obtain 240g polyhydroxynaphthalene compound, M n =410, M w = 670, D = M w / M n = 1.63.
[0040] In another reaction flask equipped with a thermometer, a constant flow pump, a stirrer, a water separator and a vacuum system, add 152g of the polyhydroxynaphthalene compound and 850mL of epichlorohydrin obtained above, stir and dissolve, and then continuously within 6 hours Add 75g of 48% sodium hydroxide aqueous soluti...
Embodiment 2
[0042] Add 216g of 1-naphthol, 72g of 2-naphthol, 48g of paraformaldehyde, and 800mL of toluene into a reaction flask equipped with a thermometer, a constant flow pump, a condenser and a stirrer, heat to 70°C, and slowly add 4.2g of A mixture of citric acid and 1.9 g of p-toluenesulfonic acid. After the addition, the temperature was raised to 100°C, and the reaction was refluxed for 10 hours. After the reaction was completed, neutralize the reaction mixture with 10% aqueous sodium carbonate solution, wash to neutrality with distilled water, and remove solvent, water and unreacted monomers in the organic phase under reduced pressure to obtain 295g polyhydroxynaphthalene compound, M n =819, M w = 1273, D = M w / M n = 1.55.
[0043] In another reaction flask equipped with thermometer, constant flow pump, stirrer, water separator and vacuum system, add the polyhydroxynaphthalene compound obtained above 150g, all the other operating steps are with embodiment 1, obtain 187g epox...
Embodiment 3
[0045] In a reaction flask equipped with a thermometer, a constant flow pump, a condenser and a stirrer, add 288g of 1-naphthol, 800mL of toluene, 2.91g of phosphotungstic acid, and 3.8g of p-toluenesulfonic acid, heat to 50°C, and slowly add 69.6 g of acetone. After the addition, the temperature was raised to 70°C, and the reaction was refluxed for 3 hours. After the reaction was completed, the reaction mixture was neutralized with 10% aqueous sodium hydroxide solution, washed with distilled water to neutrality, and the solvent, water and unreacted monomers in the organic phase were removed under reduced pressure to obtain 230 g of polyhydroxynaphthalene compounds.
[0046] In another reaction flask equipped with thermometer, constant flow pump, stirrer, water separator and vacuum system, add the polyhydroxynaphthalene compound and 900mL epichlorohydrin obtained above 165g, and all the other operating steps are the same as in Example 1, to obtain 205g epoxy resin of the pres...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com