Covalent organic framework material and preparation method and application thereof in fluorescence sensor
A covalent organic framework, fluorescence sensor technology, applied in luminescent materials, analytical materials, fluorescence/phosphorescence, etc., can solve the problems of sensitivity and reusability, poor chemically unstable excitons migration, etc., and achieve excellent thermal stability. , excellent stability, high fluorescence intensity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
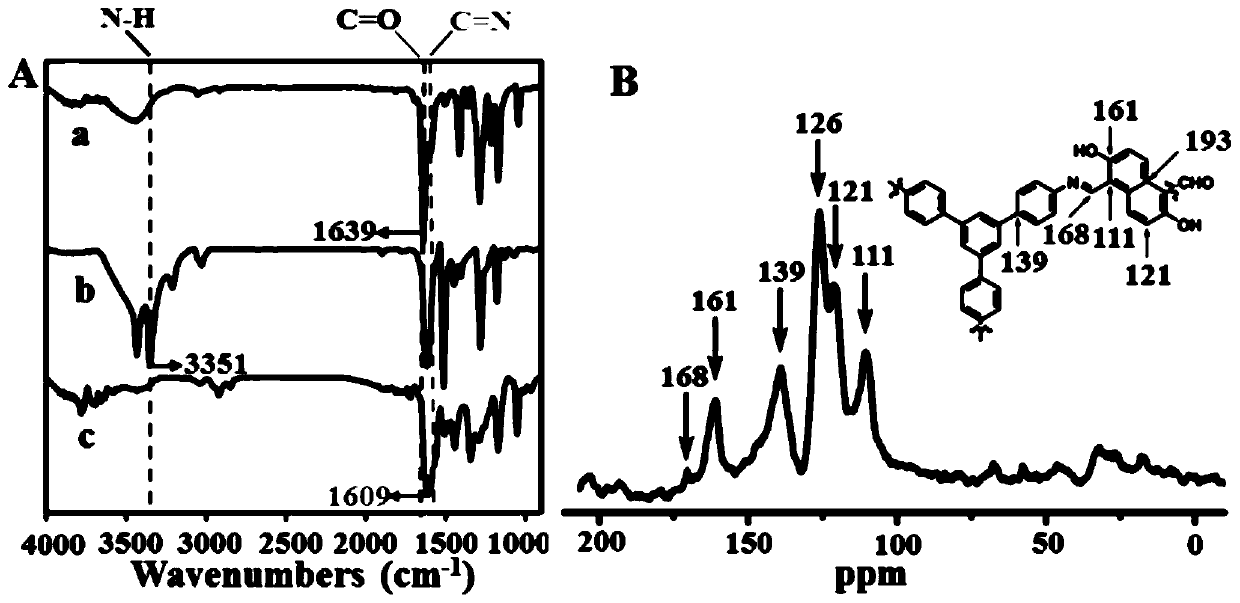
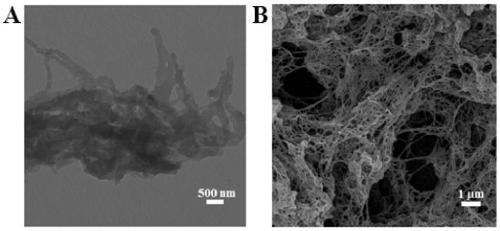
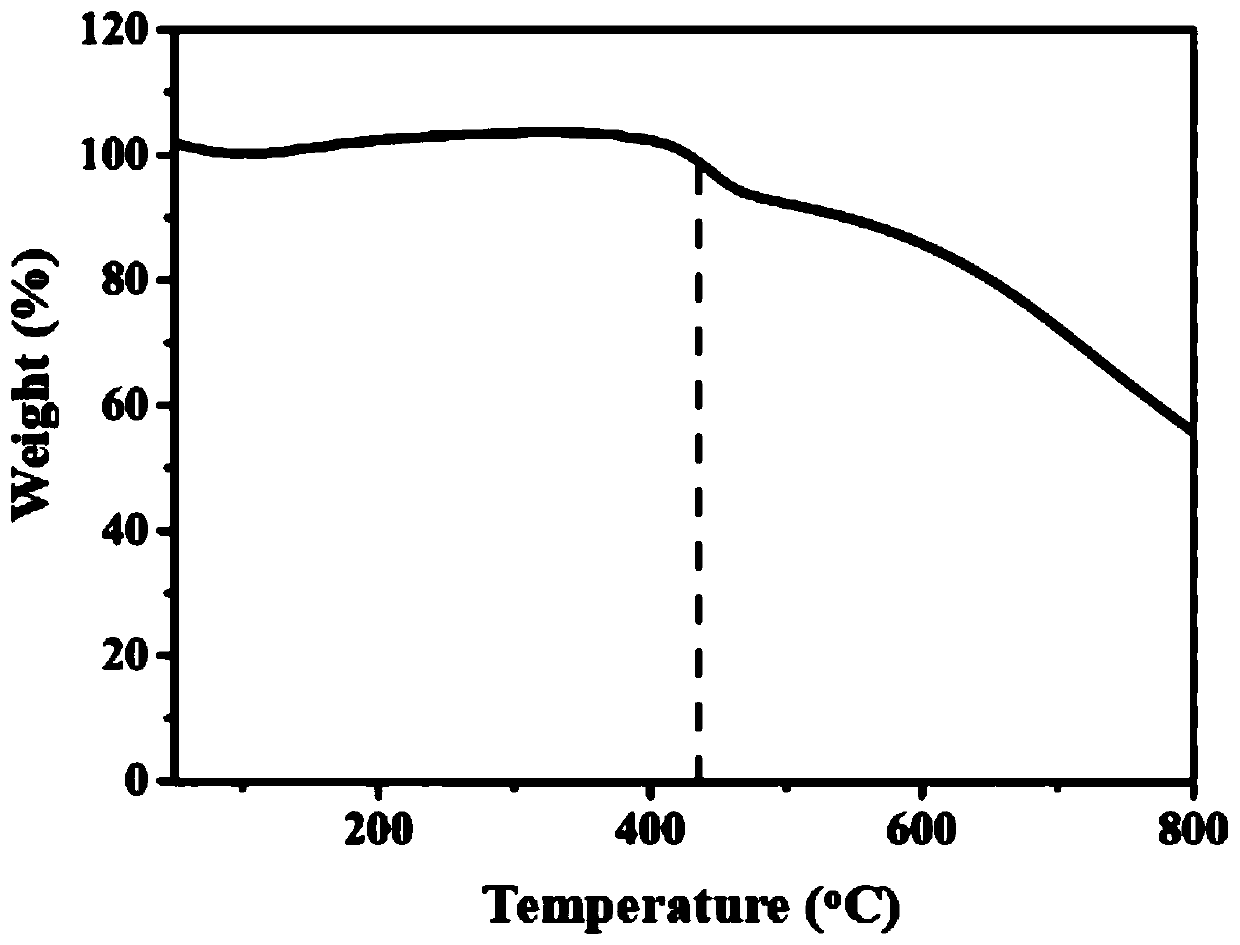
[0075] As mentioned above, COFs as chemical sensors need to have high emission efficiency, good crystallinity and chemical stability, but the corresponding explorations in these aspects are far from enough. Therefore, the present invention provides a covalent organic framework material, its preparation method and its application in a fluorescence sensor; the present invention will be further described with reference to the accompanying drawings and specific embodiments.
[0076] It should be noted that, in the following examples, the reagents and consumables are shown in Table 1, wherein, unless otherwise specified, all reagents are of analytical grade and used directly without further purification. The instruments are shown in Table 2.
[0077] Table 1
[0078]
[0079]
[0080] Table 2
[0081]
Embodiment 1
[0083] A preparation method of a covalent organic framework material (COFs-DT), comprising the steps of:
[0084] 1. The preparation of DHNDA, including:
[0085] (1) Under magnetic stirring, dissolve 1.65g of paraformaldehyde and 0.03g of NaOH with 15mL of absolute ethanol;
[0086] (2) In a low-temperature cold bath, set the temperature to 0°C, add an aqueous solution of dimethylamine (the mass ratio of dimethylamine:NaOH is 100:1) and step (1) into a 50mL round-bottomed flask to finally obtain The solution was stirred for 0.5h. Then dissolve 3.3g of 2,6-dihydroxynaphthalene with 21mL of absolute ethanol and add to the system, react for 1h; filter with a funnel to obtain a white solid, wash the filter cake with absolute ethanol several times, and put the filter cake in vacuum to dry Oven-dried for 6 hours to obtain an intermediate product (Mannich base);
[0087] (3) Weigh 1.72g of the intermediate product Mannich base and 2.46g of hexamethylenetetramine into 38mL of acet...
Embodiment 2
[0092] A preparation method of a covalent organic framework material (COFs-DT), comprising the steps of:
[0093] 1. The preparation of DHNDA, including:
[0094] (1) Under magnetic stirring, dissolve 1.7g of paraformaldehyde and 0.05g of NaOH with 25mL of absolute ethanol;
[0095] (2) In a low-temperature cold bath, set the temperature to 0°C, add aqueous dimethylamine solution (the mass ratio of dimethylamine:NaOH is 90:1) and step (1) to a 50mL round-bottomed flask to finally obtain The solution was stirred for 0.5h. Dissolve 5.75g of 2,6-dihydroxynaphthalene in 40mL of absolute ethanol and add to the system, react for 1h; filter with funnel to obtain a white solid, wash the filter cake with absolute ethanol several times, and put the filter cake in vacuum to dry Oven-dried for 6 hours to obtain an intermediate product (Mannich base);
[0096] (3) Weigh 1.5g of the intermediate product Mannich base and 2.2g of hexamethylenetetramine into 40mL of acetic acid (content: 81...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com