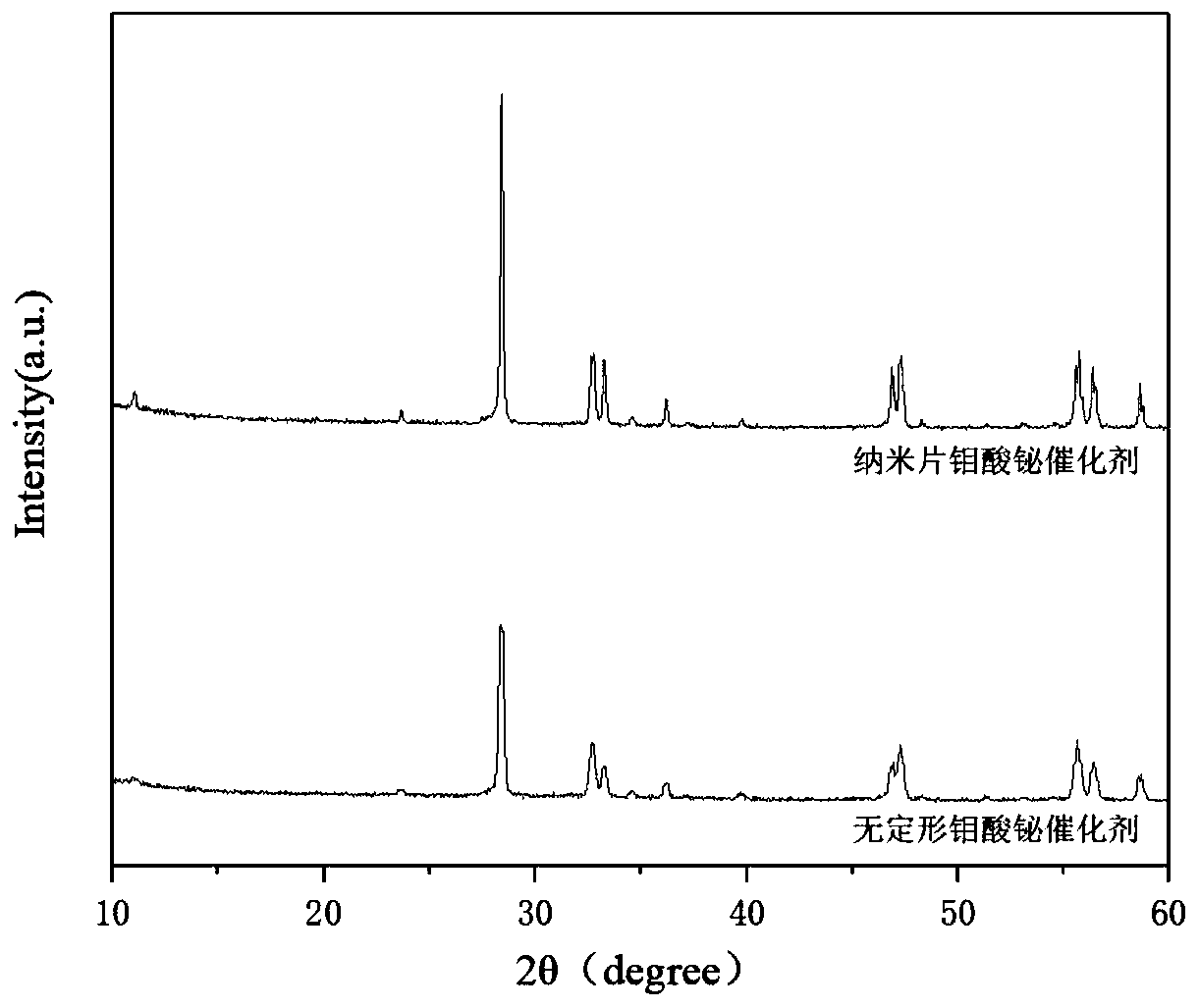
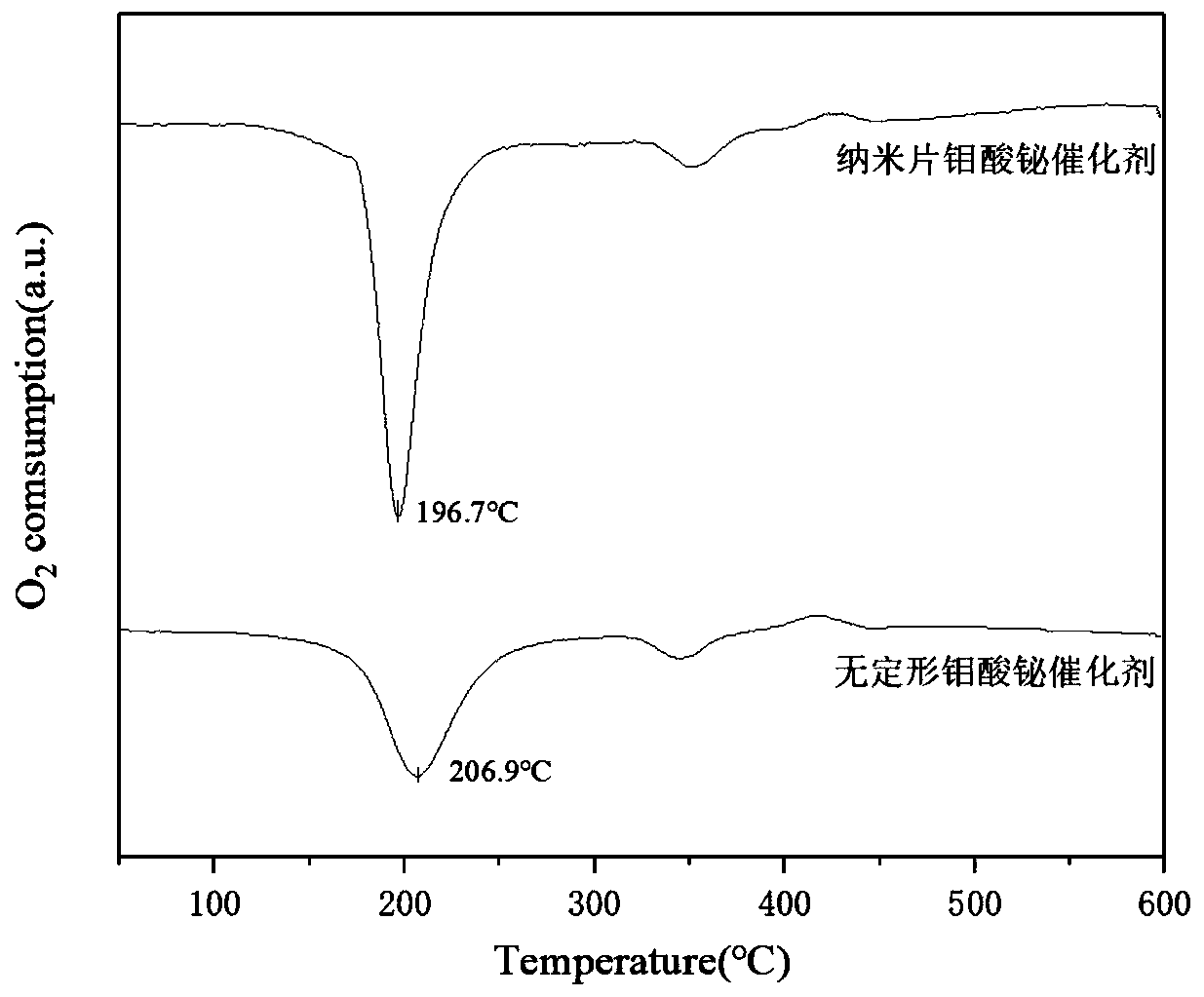
Application of Bismuth Molybdate Catalyst with Nanosheet Structure in Catalytic Synthesis of 1,3-Butadiene
A technology of nanosheets and catalysts, which is applied in the field of bismuth molybdate catalysts and catalytic synthesis 1. It can solve problems that have not been studied before, and achieve excellent effects, good stability, and simple processes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Catalyst preparation process:
[0027] Add 1.2125g Bi(NO 3 ) 3 ·5H 2 Dissolve O in 40ml deionized water, record as solution A, add 0.2207g(NH 4 ) 6 Mo 7 O 24 ·4H 2 O is dissolved in 10ml of deionized water and marked as solution B. Under magnetic stirring, transfer the A and B solutions to a 100ml container lined with polytetrafluoroethylene. After fully stirring, adjust the pH of the mixed solution to 5. Continue to stir for half an hour. The container was sealed and placed in an oven for hydrothermal reaction. The hydrothermal reaction temperature was 180°C and the time was 18h. The reaction product is collected, centrifuged, washed, and dried, and then calcined in a muffle furnace at 500° C. for 4 hours, cooled and then ground and sieved to obtain 40-60 mesh bismuth molybdate nanoplate catalyst.
[0028] Oxidative dehydrogenation reaction process:
[0029] 1 g of the above catalyst was filled into a stainless steel reactor with an inner diameter of 8 mm, and 1-butene was ...
Embodiment 2
[0032] Catalyst preparation process:
[0033] Add 1.2125g Bi(NO 3 ) 3 ·5H 2 Dissolve O in 50ml deionized water, record as solution A, add 0.2207g(NH 4 ) 6 Mo 7 O 24 ·4H 2 O is dissolved in 10ml of deionized water and marked as solution B. Under magnetic stirring, transfer the A and B solutions to a 100ml container lined with polytetrafluoroethylene. After fully stirring, adjust with 3mol / L NaOH solution The pH of the mixed solution was 6, and the stirring was continued for half an hour. The container was sealed and placed in an oven for hydrothermal reaction. The hydrothermal reaction temperature was 180°C and the time was 20h. The reaction product is collected, centrifuged, washed, and dried, and then calcined in a muffle furnace at 500° C. for 4 hours, cooled and then ground and sieved to obtain 40-60 mesh bismuth molybdate nanoplate catalyst.
[0034] Oxidative dehydrogenation reaction process:
[0035] 1 g of the above catalyst was filled into a stainless steel reactor with an ...
Embodiment 3
[0038] Catalyst preparation process:
[0039] Add 1.2125g Bi(NO 3 ) 3 ·5H 2 O is dissolved in 55ml deionized water, and it is recorded as solution A, and 0.2207g(NH 4 ) 6 Mo 7 O 24 ·4H 2 O is dissolved in 5ml of deionized water and marked as solution B. Under the condition of magnetic stirring, transfer the A and B solutions to a 100ml container lined with polytetrafluoroethylene. After fully stirring, adjust the pH of the mixed solution with ammonia 6. Continue to stir for half an hour. The container was sealed and placed in an oven for hydrothermal reaction. The hydrothermal reaction temperature was 160°C and the time was 24h. The reaction product is collected, centrifuged, washed, and dried, and then calcined in a muffle furnace at 550°C for 3 hours, cooled and then ground and sieved to obtain a 40-60 mesh bismuth molybdate nanoplate catalyst.
[0040] Oxidative dehydrogenation reaction process:
[0041] 1 g of the above catalyst was filled into a stainless steel reactor with an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com