Test method of hot corrosion performance of nickel-based single-crystal superalloy
A high-temperature alloy and nickel-based single crystal technology, which is applied in the fields of weather resistance/light resistance/corrosion resistance, measuring devices, instruments, etc., can solve the problems of reducing the service life and reliability of turbine blades, and blade failure, so as to improve accuracy and Effects of repeatability and reliability improvement
Inactive Publication Date: 2019-08-16
NORTHWESTERN POLYTECHNICAL UNIV
View PDF5 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The hot corrosion of the alloy will accelerate the initiation and expansion of cracks on the surface of the turbine blade. When the hot corrosion of the
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
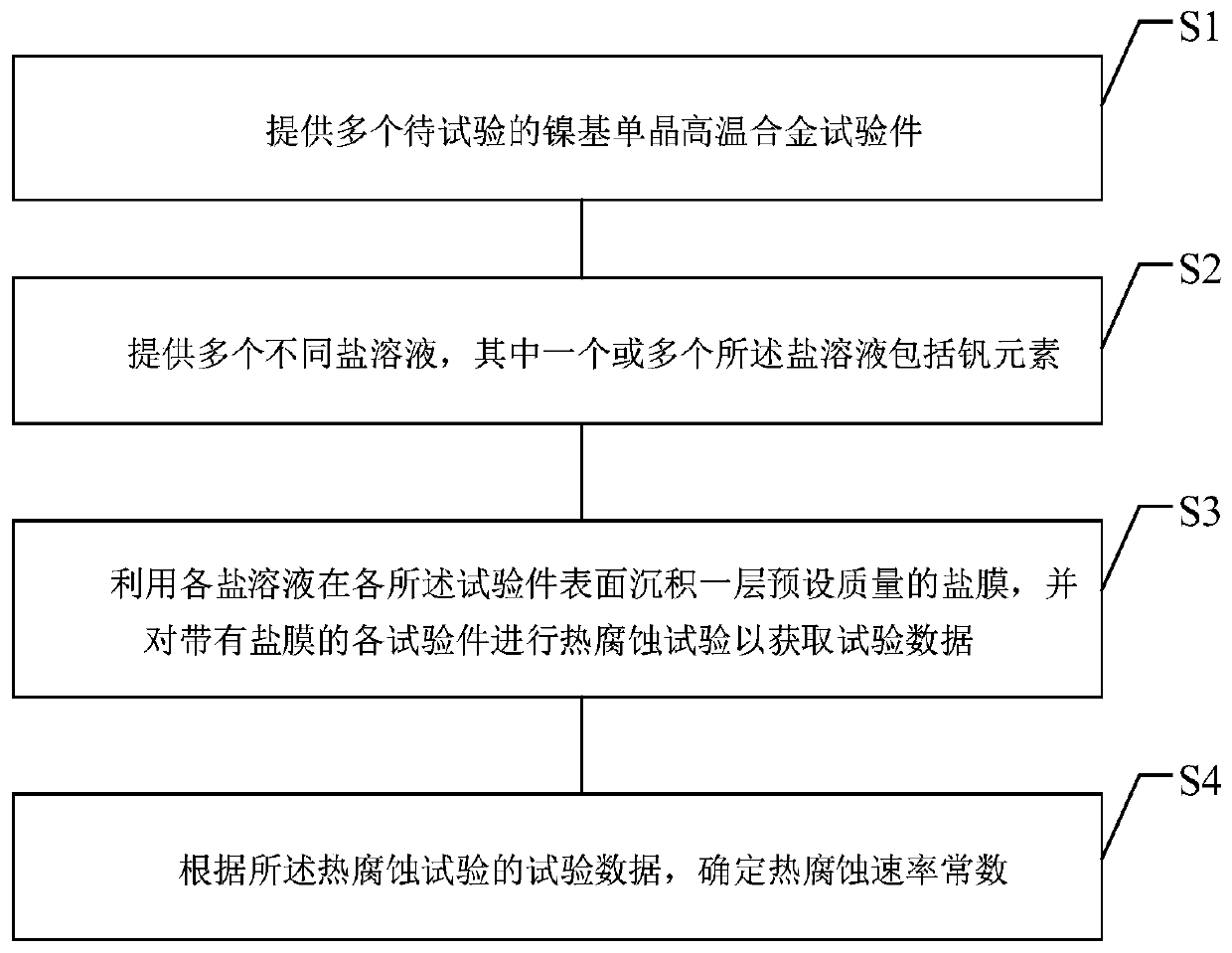
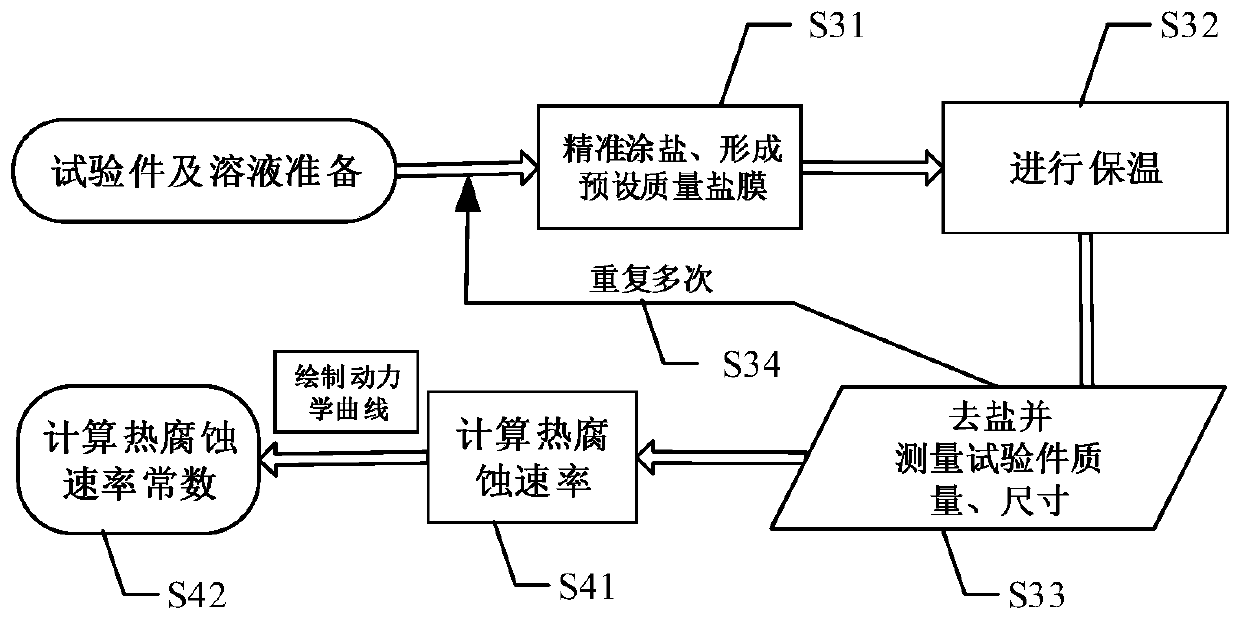
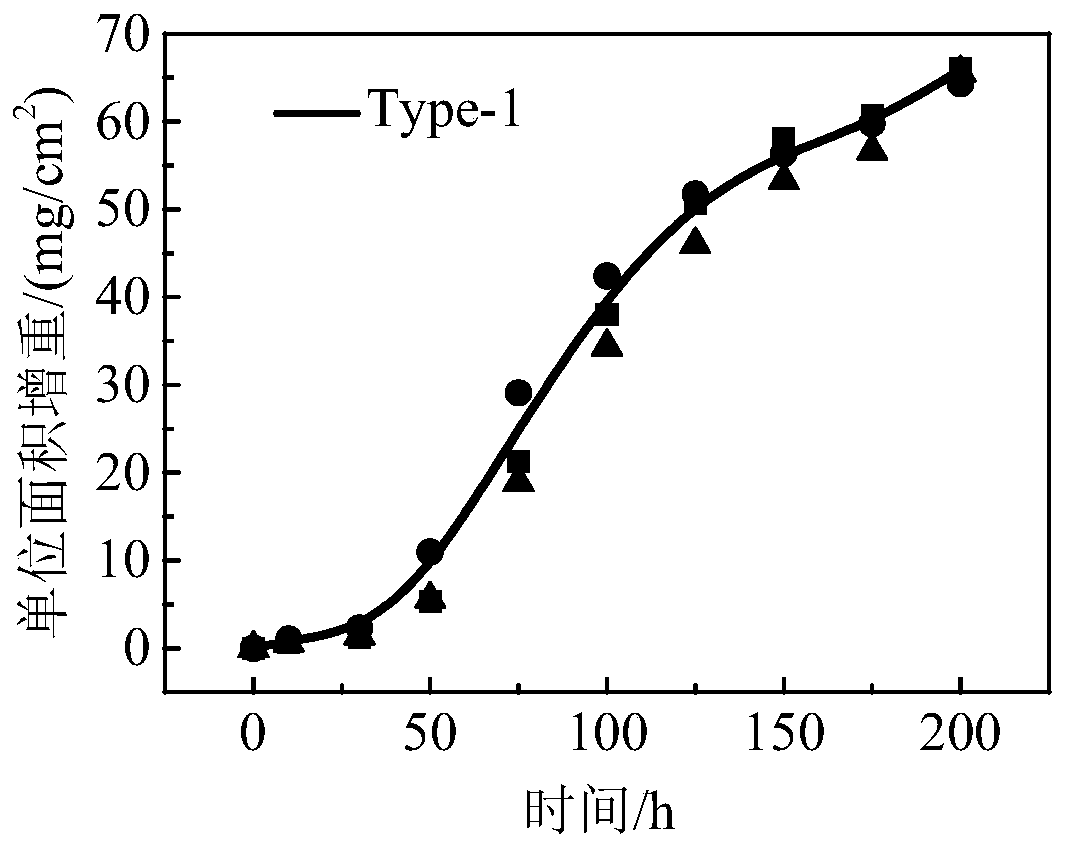
The invention relates to a test method of hot corrosion performance of a nickel-based single-crystal superalloy and belongs to the field of hot corrosion testing. The test method comprises the steps that multiple to-be-tested nickel-base single-crystal superalloy test pieces are provided; multiple different saline solutions are provided, wherein one or more saline solutions contain vanadium elements; each saline solution is utilized to deposit a saline film with a preset mass on the surface of each test piece, and a hot corrosion test is performed on each test piece provided with the saline film to acquire test data; and according to the test data of the hot corrosion test, a hot corrosion rate constant is determined. Through the test method, on the one hand, the saline solutions containing the vanadium elements are supplemented to perform the hot corrosion test on the test pieces, a test environment more approximate to a superalloy actual service environment is provided, and the reliability of the test is improved; and on the other hand, the saline film content on the surface of each test piece is precisely controlled, so that the accurate test data is acquired, quantitative analysis of the hot corrosion performance is benefited, and the accuracy and repeatability of the test are improved.
Description
technical field [0001] The disclosure relates to the field of hot corrosion tests, in particular to a test method for hot corrosion performance of nickel-based single crystal superalloys. Background technique [0002] Nickel-based single crystal superalloys have excellent high-temperature mechanical properties, oxidation resistance and corrosion resistance, and are widely used in the manufacture of aviation or industrial engine turbine blades. In order to obtain more excellent creep performance and good structural stability, the addition of Al (aluminum), Cr (chromium), etc., which can produce protective oxide elements such as Al (aluminum) and Cr (chromium), in the manufacture of a new generation of superalloys has reduced the content of the alloy at a higher It exhibits relatively poor oxidation resistance and thermal corrosion resistance at the service temperature. [0003] Hot corrosion refers to an accelerated oxidation phenomenon of nickel-based single crystal alloys ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): G01N17/00
CPCG01N17/006
Inventor 温志勋岳珠峰杨艳秋赵彦超王佳坡谷淑宁
Owner NORTHWESTERN POLYTECHNICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com