Application of teniposide in anti-mycobacterium tuberculosis drugs
A technology of mycobacterium tuberculosis and teniposide, which is applied in the field of bioengineering, can solve the problems that tuberculosis drugs cannot meet the requirements of patients and tuberculosis prevention and control, and achieve the effects of increasing reuse value, shortening the course of treatment, and saving treatment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
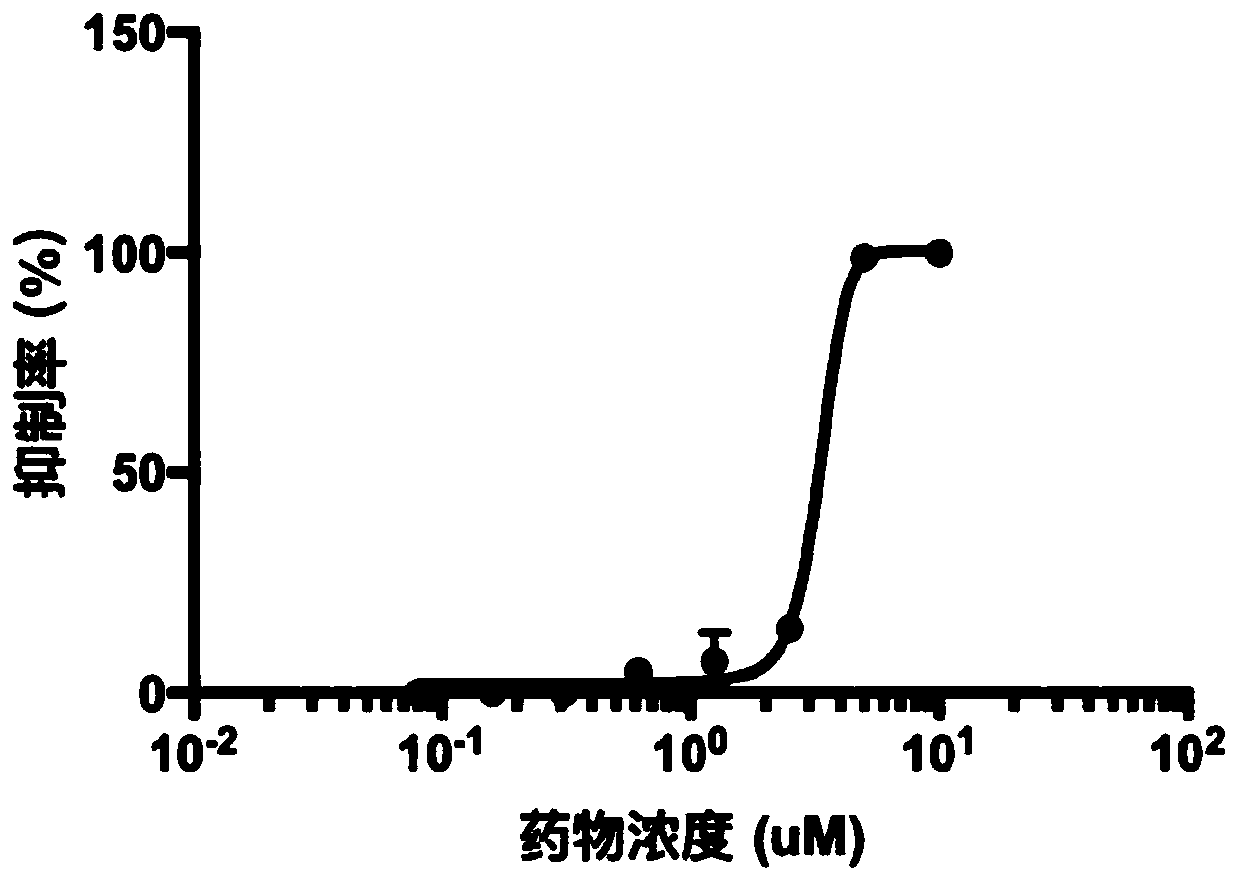
[0024] In vitro bactericidal activity assay
[0025] 1. Preparation of drugs with different concentrations
[0026] Under sterile conditions, use DMSO to prepare teniposide into a solution with a concentration of 10uM. After fully dissolving, use DMSO to dilute the solution into eight concentration gradients, so that the drug concentrations are: 1uM, 500uM, 250uM, 125uM , 62.5uM, 31.25uM, 15.6uM, 7.8uM are stored at -80℃ for later use;
[0027] 2. Preparation of Mycobacterium tuberculosis (Mtb)
[0028] Culture Mycobacterium tuberculosis H37Rv (No. ATCC27294) in 7H9-OADC medium, dilute Mtb in the logarithmic growth phase (OD600=0.6~0.8) to OD600=0.01, spread 99uL / well on a 96-well cell culture plate middle;
[0029] Add 1ul of the drug with the above concentration gradient to the cultured bacteria, so that the final concentration of the drug is: 10uM, 5uM, 2.5uM, 1.25uM, 625uM, 312uM, 156uM, 78uM, and add 1ul of DMSO to the above eight groups of solutions with the same conc...
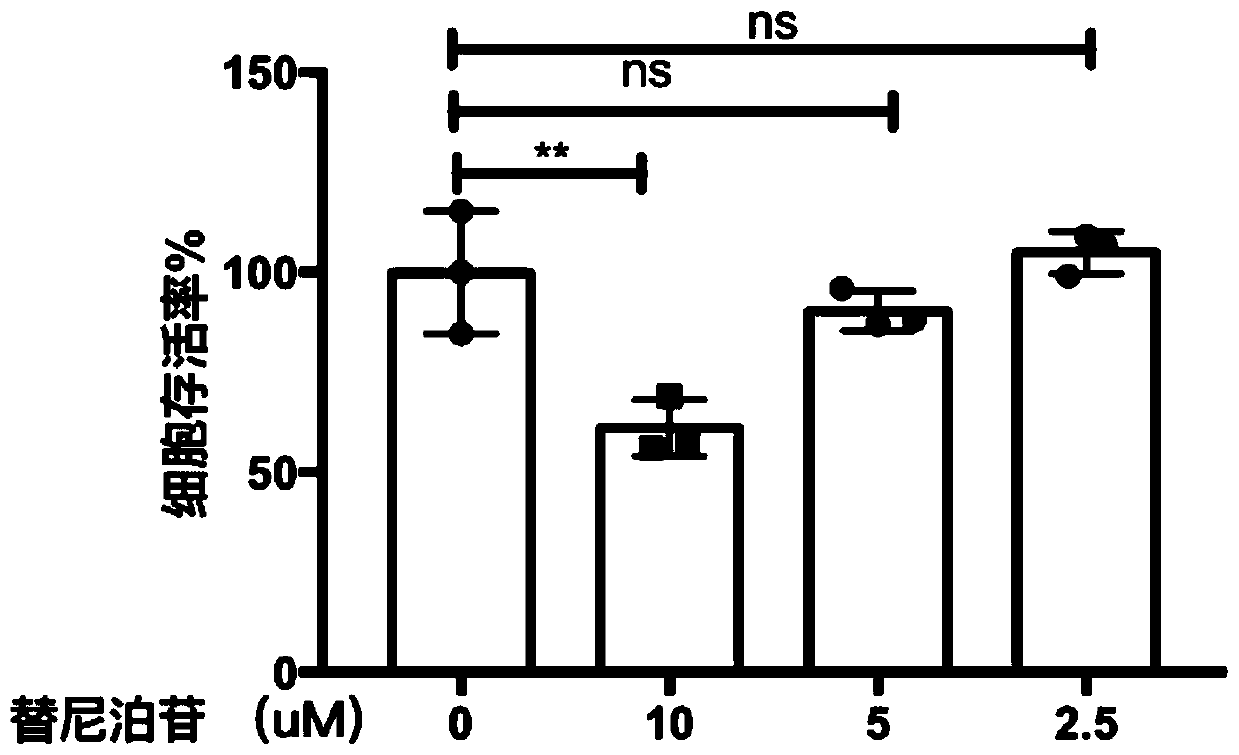
Embodiment 2
[0037] 1. Differentiation of U937 macrophages (No. BNCC100967): Suspended U937 macrophages were cultured in RPMI-1640 medium containing 10% FBS, and placed at a temperature of 37°C, containing 5% CO 2 Cultured in a cell incubator, added PMA with a final concentration of 20ng / ml, cultured overnight, differentiated into macrophages, washed twice with PBS, digested with trypsin, resuspended, added to a 96-well cell culture plate , so that the number of cells per well is 1x104;
[0038] 2. Determination of cell survival rate: After culturing the above-mentioned U937 macrophages for 24 hours, add different concentrations of drugs to make the final drug concentrations 10uM, 5uM, and 2.5uM. The experiment was repeated three times for each concentration, and DMSO was used as a parallel control In the experiment, DMSO with the same volume as the drug was added to the control group, and the experiment was repeated three times. After 48 hours, 10ul WST-1 solution was added to each well, ...
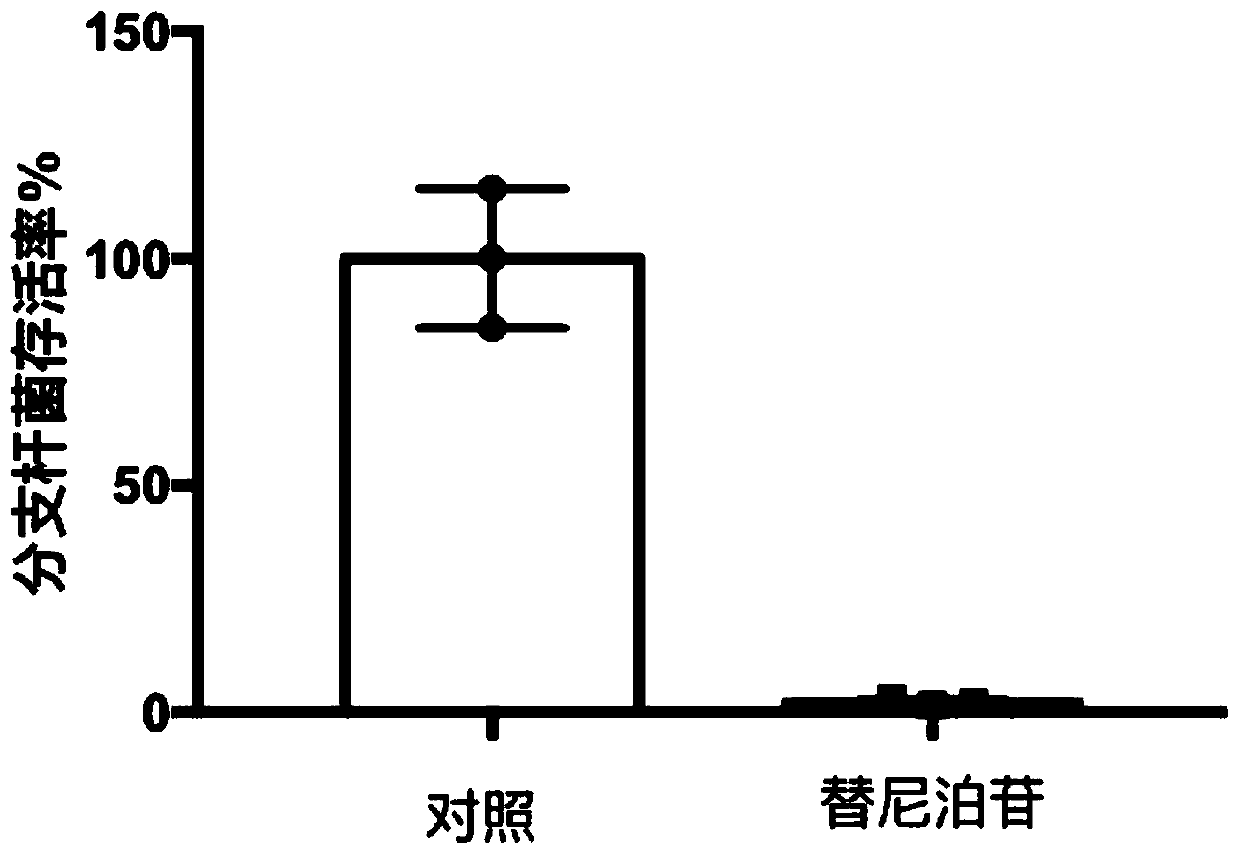
Embodiment 3
[0045] 1. Differentiation of U937 macrophages: Suspended U937 macrophages were cultured in RPMI-1640 medium containing 10% FBS, and placed at a temperature of 37°C, containing 5% CO 2 Cultured in a cell incubator, added PMA with a final concentration of 20ng / ml, cultured overnight, differentiated into macrophages, washed twice with PBS, digested with trypsin, resuspended, added to a 96-well cell culture plate , so that the number of cells per well is 1x104;
[0046] 2. Determination of intracellular bactericidal activity: After the above-mentioned U937 macrophages were cultured for 24 hours, teniposide was added to make the final concentration 5uM, and the experiment was repeated three times for each concentration. DMSO was used as a parallel control experiment, and teniposide was added to the control group. DMSO with the same volume as the drug was repeated 3 times. After 4 hours, the medium was sucked off, washed twice with PBS, fresh medium was added for 72 hours, and the l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com