Generating atrial and ventricular cardiomyocyte lineages from human pluripotent stem cells
A technique for ventricular cardiomyocytes and atrial cardiomyocytes, applied in the field of generating atrial cardiomyocytes and ventricular cardiomyocyte lineages from human pluripotent stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
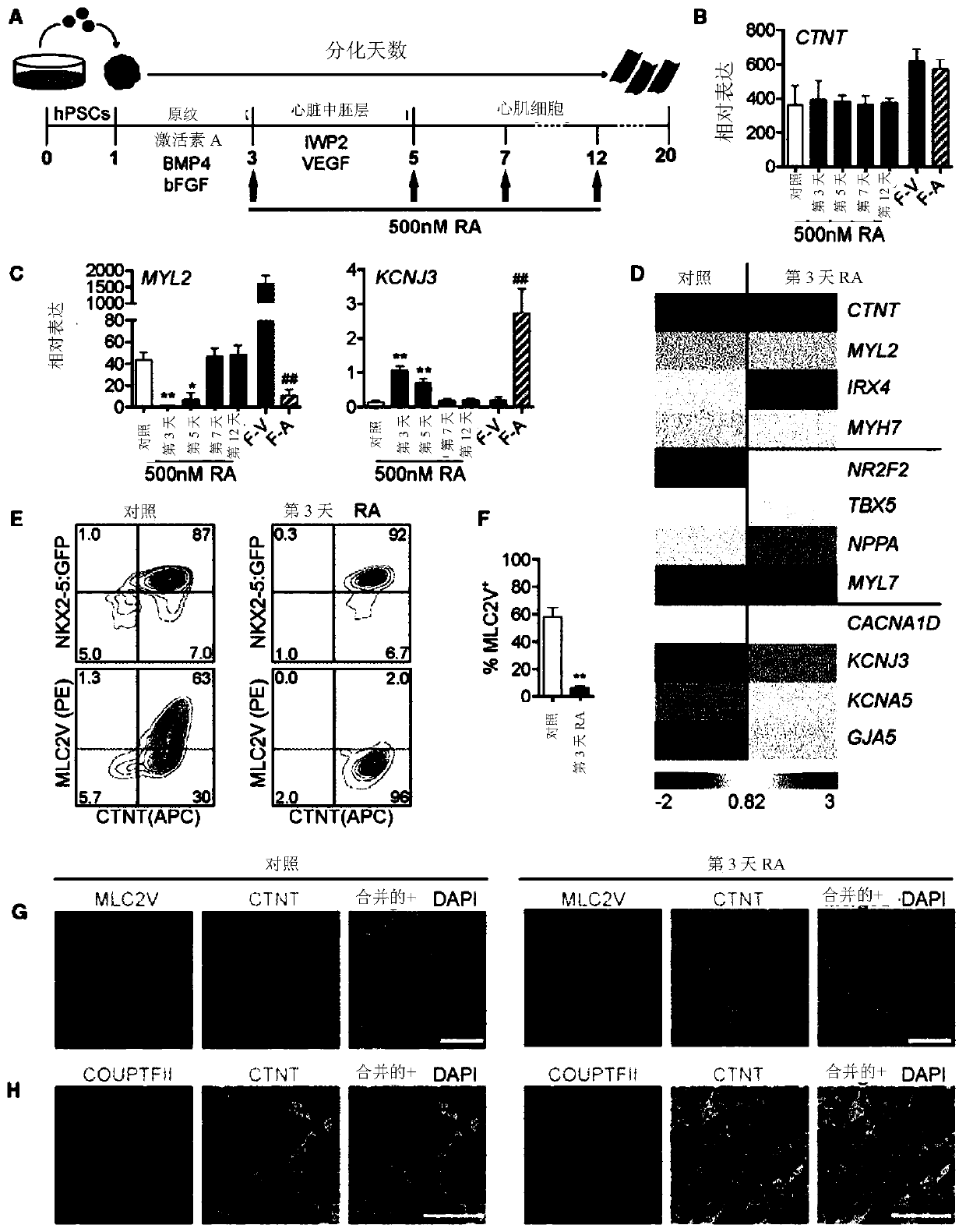
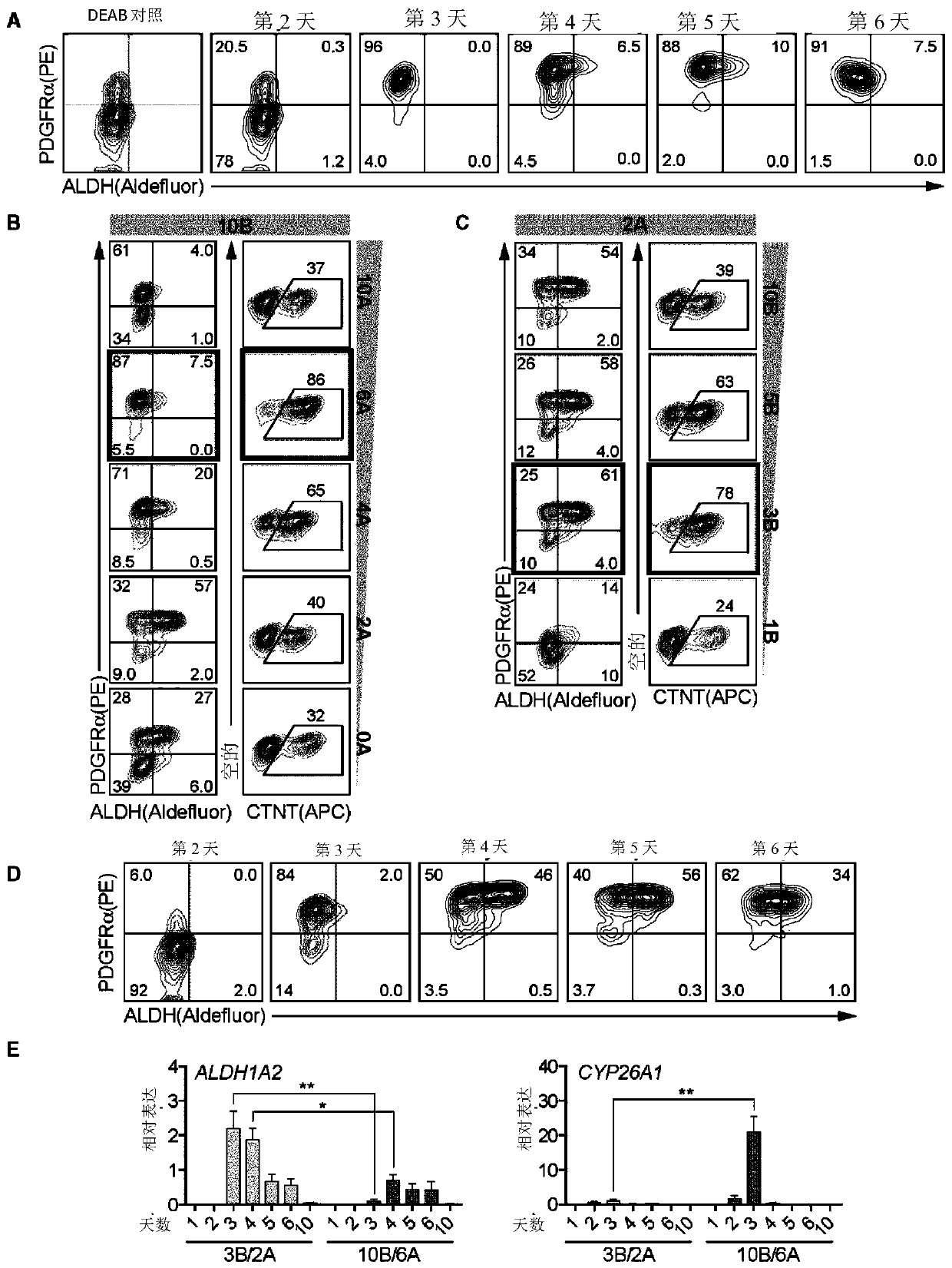
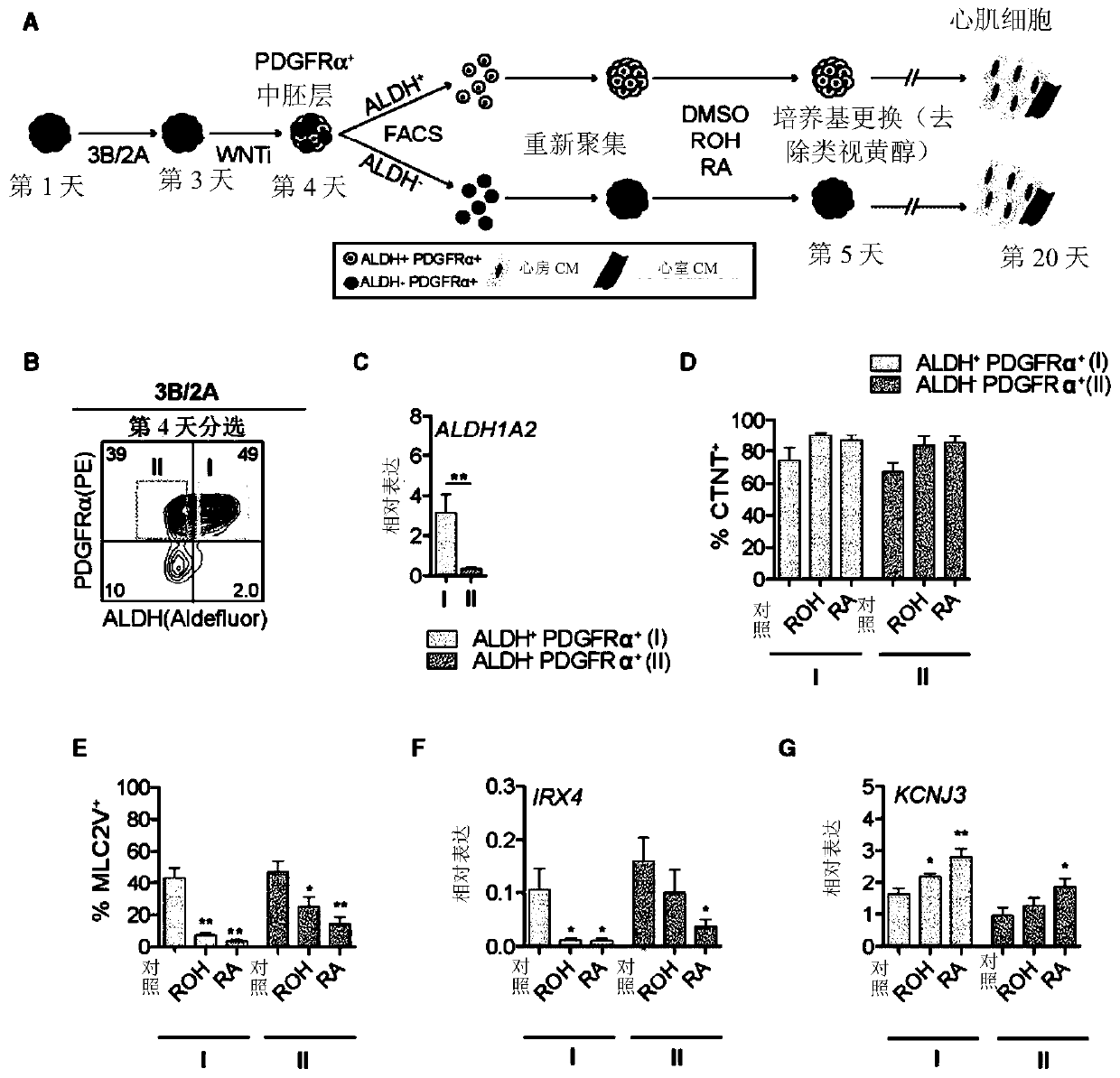
[0108] Methods and Results
[0109] Human pluripotent stem cell lines can be cultured as previously described (eg Kennedy et al., 2007). For differentiation into cardiac lineages, established protocols such as those described in Kattman et al., 2011 can be used. Various modifications to the procedure are possible, including those described in WO2016131137. In one example, 80% confluent hPSC cultures can be dissociated into single cells, suspended in StemPro-34 medium containing 1 ng / ml BMP4 and 10 μM ROCK inhibitor, and incubated on an orbital shaker for 18 hours to generate embryoid bodies (EBs). The next day (Day 1 of differentiation), EBs can be transferred to mesoderm induction medium: StemPro containing a set concentration of BMP4, and a set concentration of Activin A as described further herein, and 5 ng / ml bFGF -34. On day 3 of differentiation, the EBs can be washed once with IMDM and suspended in cardiac induction medium: in one example, cardiac induction medium ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com