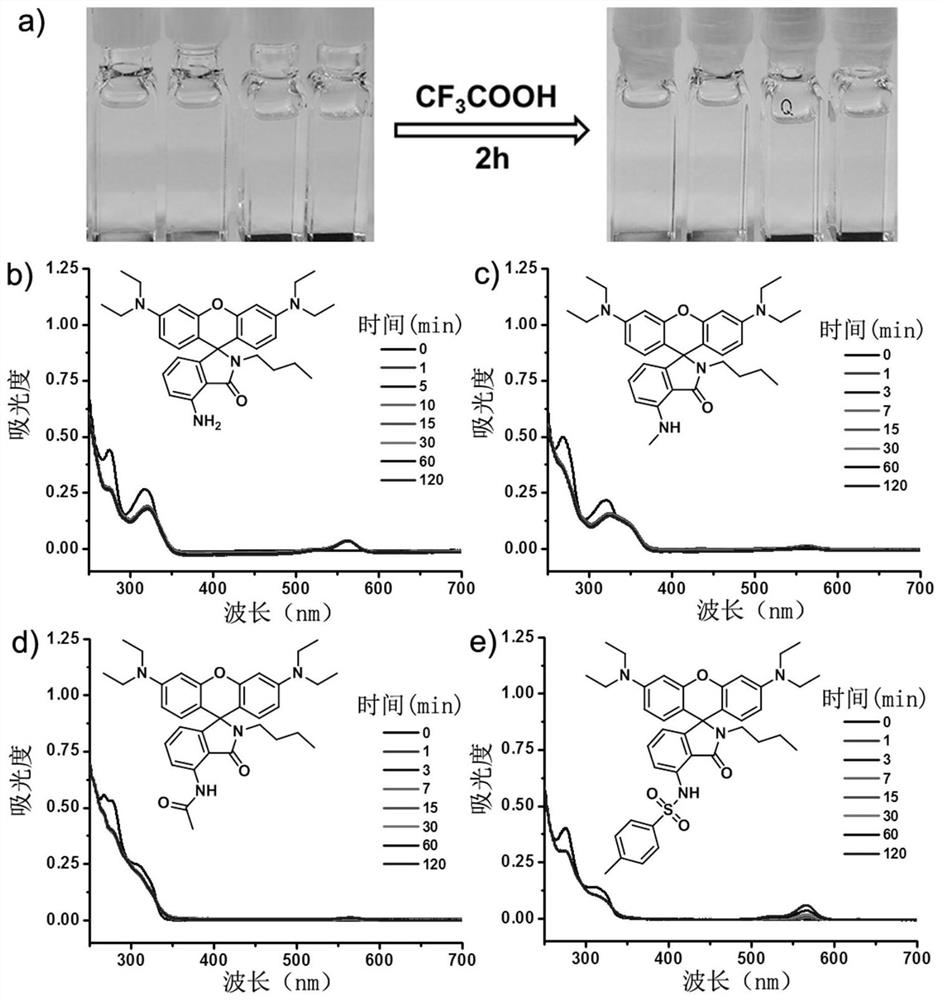
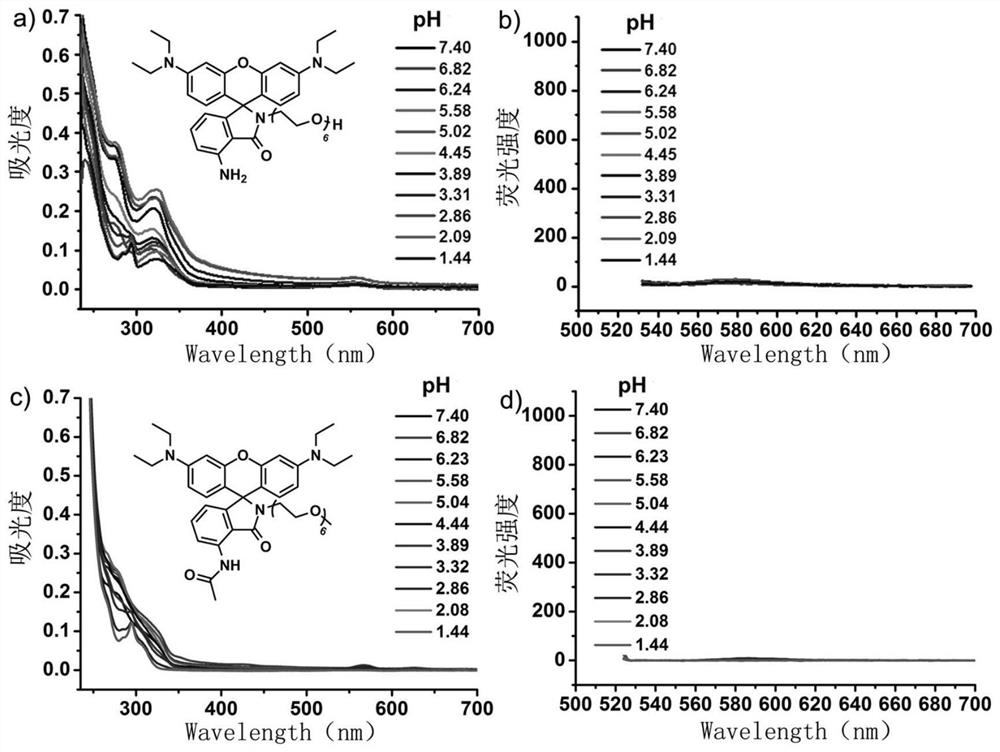
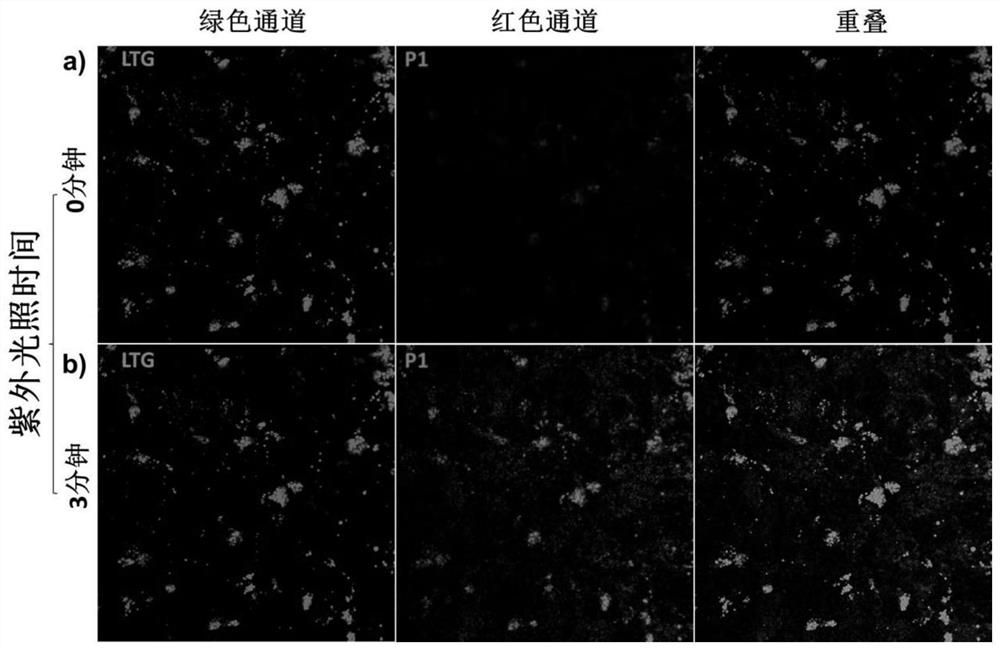
A kind of acid-resistant light-controlled fluorescent molecular switch and its synthesis method and application
A technology of fluorescent molecule and synthesis method, which is applied in the field of acid-resistant light-controlled fluorescent molecular switch and its synthesis, can solve the problems of switching interference, performance failure of light-controlled molecular switch, inability of fluorescent probe to be applied to super-resolution fluorescence imaging, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] When R 1 = R 2 = R 3 = R 4 =C 2 h 5 , R 5 =H, X=O, Y=H, Z=C 4 h 9 When, its molecular (P1) synthetic route and product structure are as follows:
[0034]
[0035] Synthesis steps and characterization: 3-Nitrorhodamine (5mmol, 2.4g) and n-butylamine (20mmol, 1.4g) were dissolved in absolute ethanol (50mL). The temperature was raised to 78°C for reflux, and after stirring for 8 hours, the solvent was evaporated under reduced pressure, and the product was separated and purified by silica gel chromatography (petroleum ether / ethyl acetate, 8:1v / v), and the obtained light yellow powder (2.6g, 95% ). Then all the powder was dissolved in methanol / dichloromethane (50mL, 3:1v / v) mixed solvent, under hydrogen atmosphere, by palladium carbon (0.21g, 10%wt) catalytic reduction, the filtrate was taken by suction filtration, and the The final product (2 g, 98%) was obtained as a white powder after removal of the solvent by autoclaving.
[0036] The product was characteri...
Embodiment 2
[0040] When R 1 = R 2 = R 3 = R 4 =C 2 h 5 , R 5 =CH 3 , X=O, Y=H, Z=C 4 h 9 When, its molecular (P2) synthesis route and product structure are as follows:
[0041]
[0042] Synthesis steps and characterization: P1 (0.25g, 0.5mmol), iodomethane (0.28g, 2mmol) and potassium carbonate (0.34g, 2.5mmol) were mixed in acetonitrile (8mL), stirred at reflux for 10 hours, cooled to room temperature and filtered The filtrate was obtained, and the solvent was evaporated under reduced pressure. The crude product was separated and purified by column chromatography (silica gel, petroleum ether / ethyl acetate, 10:1 v / v) to obtain white powder P2 (0.17 g, 65%).
[0043] The product was characterized by NMR and mass spectrometry: 1 H NMR (400MHz, CDCl 3 )δ7.23(t, J=7.9Hz, 1H), 6.75(d, J=4.9Hz, 1H), 6.57(t, J=9.3Hz, 2H), 6.49(d, J=8.1Hz, 1H) ,6.41–6.22(m,5H),3.33(q,J=7.0Hz,8H),3.04(s,2H),2.97(d,J=4.9Hz,3H),1.16(t,J=6.9Hz, 12H), 1.07(s, 4H), 0.67(t, J=6.5Hz, 3H). 13 C NMR (101MH...
Embodiment 3
[0047] When R 1 = R 2 = R 3 = R 4 =C 2 h 5 , R 5 =CH 3 CO, X=O, Y=H, Z=C 4 h 9 When, its molecular (P3) synthesis route and product structure are as follows:
[0048]
[0049] Synthesis steps and characterization: P1 (0.25g, 0.5mmol) and acetyl chloride (58mg, 0.75mmol) were mixed in dichloromethane (5mL), stirred for 2 hours, and the solvent was evaporated under reduced pressure, and the crude product was passed through column chromatography (silica gel, Petroleum ether / ethyl acetate, 8:1 v / v) was separated and purified to obtain white powder P3 (0.26 g, 95%).
[0050] The product was characterized by NMR and mass spectrometry: 1 H NMR (400MHz, CDCl 3 )δ10.60(s,1H),8.43(d,J=8.2Hz,1H),7.39(t,J=7.9Hz,1H),6.74(d,J=7.6Hz,1H),6.46(d, J=8.8Hz, 2H), 6.38(d, J=2.6Hz, 2H), 6.28(dd, J=8.9, 2.6Hz, 2H), 3.34(q, J=7.0Hz, 8H), 3.06(t, J=7.0Hz, 2H), 2.29(s, 3H), 1.17(t, J=7.0Hz, 12H), 1.12–1.02(m, 4H), 0.69(t, J=6.7Hz, 3H). 13 CNMR (101MHz, CDCl 3 )δ169.30,168.85,158.27,15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com