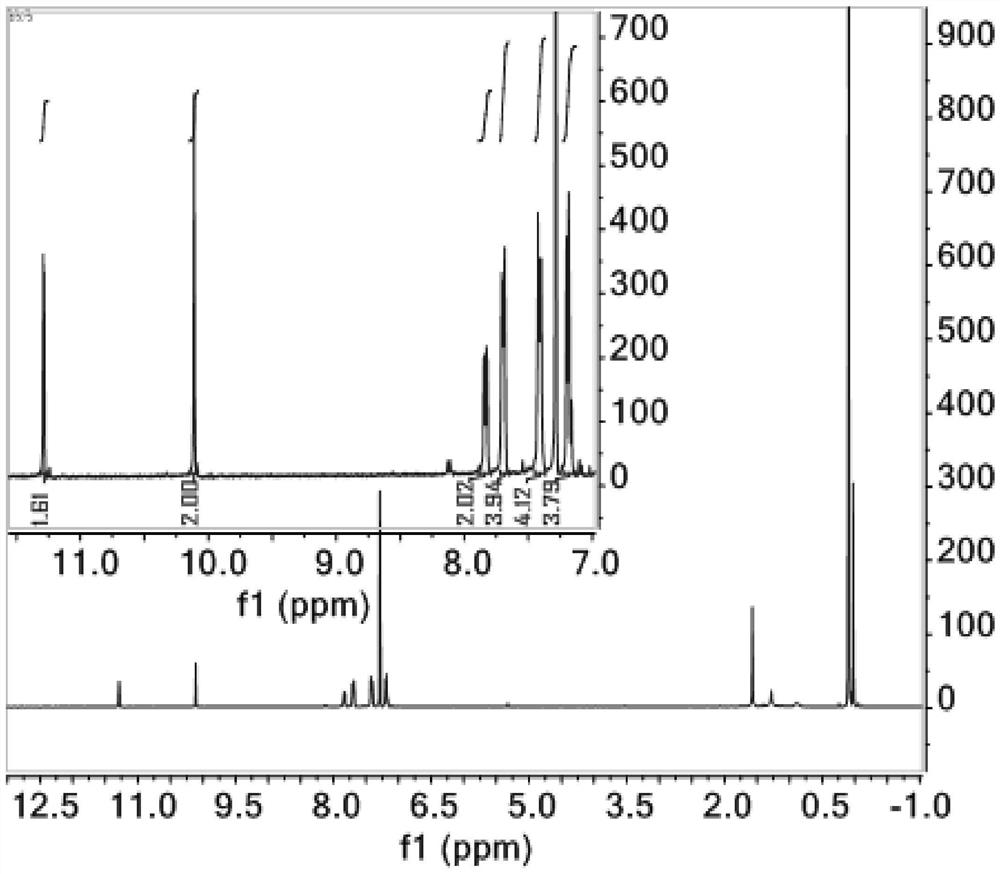
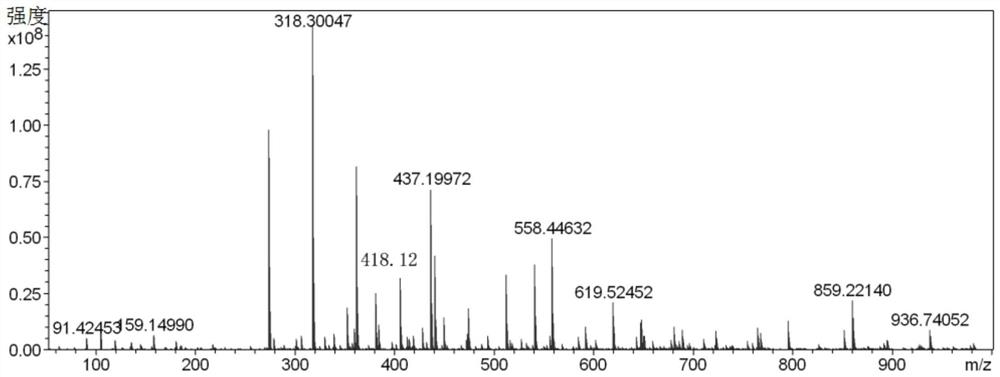
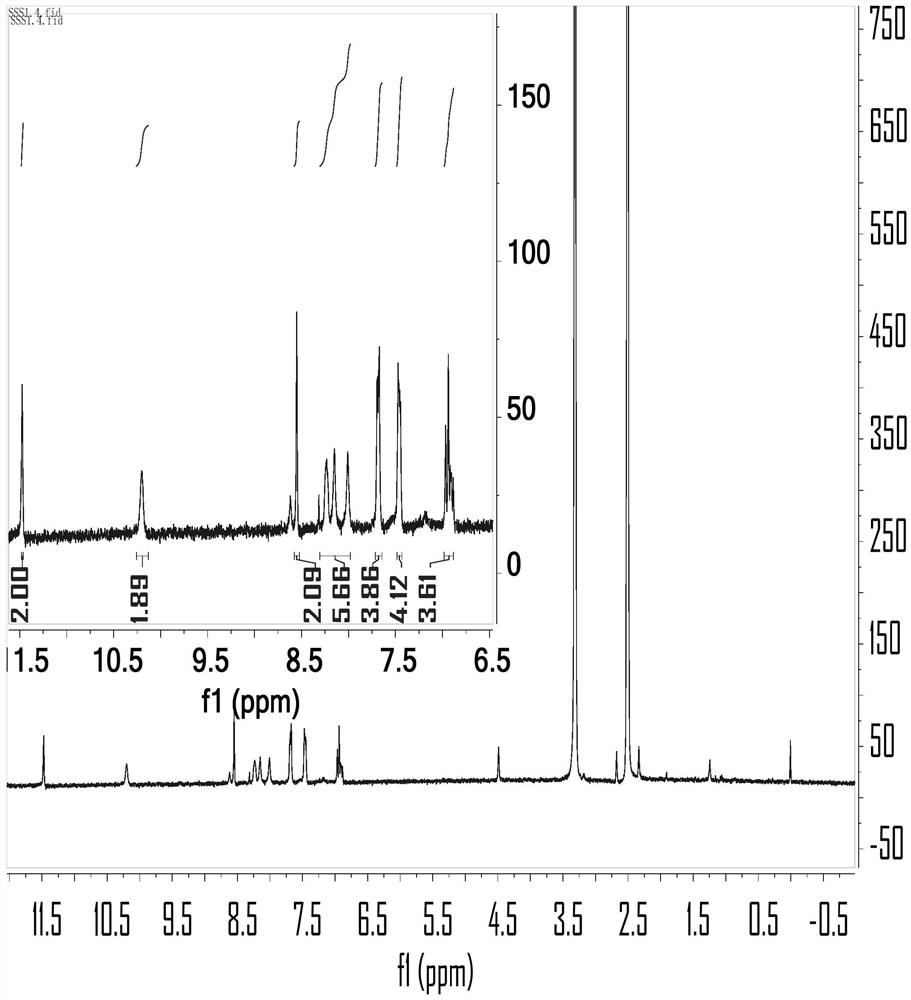
Anthracene-thiosemicarbazide derivatives and their preparation methods and applications as fluorescent probes
A technology of thiosemicarbazide and derivatives, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve the effects of less cell damage, low excitation energy, and deeper penetration depth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In a 250ml three-necked flask, add 4-bromo-2-hydroxybenzaldehyde (2.84g, 11.19mmol), 9,10-diborate anthracene (2g, 4.73mmol) dissolved in 100ml 1,4-dioxane and 24ml ethanol, K 2 CO 3 (3.92 g, 28.38 mmol) was dissolved in 48 mL of distilled water and mixed into the above solution. Then, nitrogen gas was bubbled into the mixed solution for 15 minutes, and then tetrakis(triphenylphosphine) palladium (0) (0.39 g, 1.2 mmol) was added, and nitrogen gas was bubbled in for another 5 minutes, and the reaction was carried out at 120°C in a nitrogen atmosphere. During the reaction, The progress of the reaction was tracked by spotting the plate. The developer was dichloromethane:petroleum ether=1:1. After 48 hours of reaction, the spot of raw material 9,10-diborate anthracene almost disappeared, and the reaction was stopped. After the reaction was over, the reaction solution was distilled under reduced pressure to obtain a black solid mixture, and the organic phase was extracted...
Embodiment 2
[0039] The probe molecule of Example 1 was added to DMF, and the probe molecule DMF mother solution was prepared with a concentration of 1 mM.
[0040] Add 1.97 mL DMF solution and 1 mL buffered aqueous solution (the buffer solution is Na 2 HPO 4 / NaH 2 PO 4 , pH is 7, 1 / 2, v / v), then take 30 μL probe molecule DMF mother solution (concentration: 1mM) and add it to the above-mentioned quartz cuvette, ultrasonically dissolve it, and then prepare a 10 μM probe molecule Needle molecule solution.
[0041] Similarly, add 1.7 mL DMF solution and 1 mL buffered aqueous solution (Na 2 HPO 4 / NaH 2 PO 4 , pH is 7, 1 / 2, v / v), then take 300 μL of probe molecule DMF mother solution (concentration: 1mM) and add it to the above-mentioned quartz cuvette, ultrasonically dissolve it, and then prepare a 100 μM probe molecule Needle molecule solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap