A method for synthesizing benzylamines by catalytic hydrogenation of imines
A technology for imines to catalyze hydrogen and benzylamines, which is applied in the preparation of organic compounds, preparation of amino compounds, chemical instruments and methods, etc., can solve the problems of high reaction cost, high reaction risk, poor environmental protection, etc., and meet the reaction conditions Simple, convenient operation steps, high synthesis rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
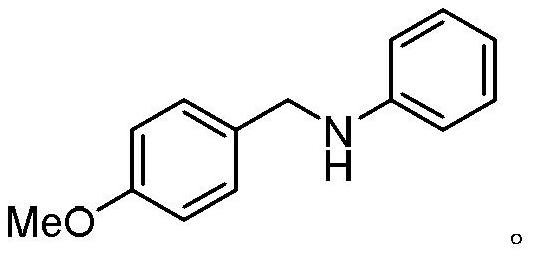
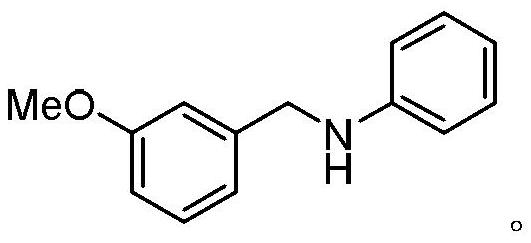
Examples
specific Embodiment approach 1
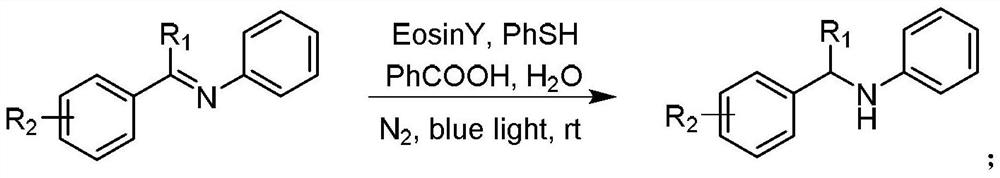
[0012] Embodiment 1: In this embodiment, the method for synthesizing benzylamine compounds by catalytic hydrogenation of imines is implemented in the following steps:
[0013] Under a nitrogen atmosphere, use imine as the reaction raw material, thiophenol as the hydrogen source, add an organic visible light catalyst, use a weak acid as a co-catalyst, and add the reaction solvent into the reaction vessel together, and carry out the reaction under the condition of visible light. After completion, the reaction system is concentrated, separated and purified to obtain benzylamine derivatives;
[0014] Wherein said organic visible light catalyst is Eosin Y (Eosin Y), Rhodamine B (Rhodamine B) or fac-Ir (ppy) 3 .
specific Embodiment approach 2
[0015] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the co-catalyst is benzoic acid, acetic acid, malic acid or citric acid.
specific Embodiment approach 3
[0016] Embodiment 3: This embodiment differs from Embodiment 1 or Embodiment 2 in that the molar ratio of imine to thiophenol is (1-2): (2-6).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com