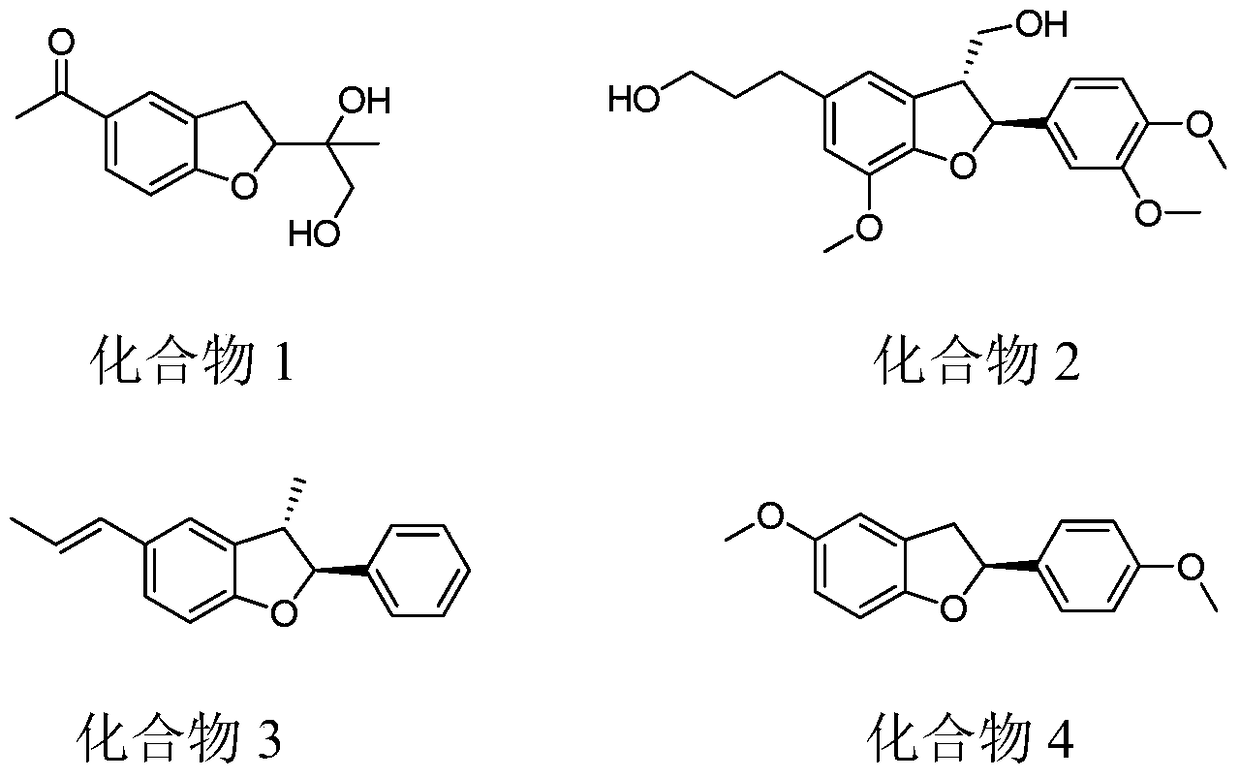
A kind of preparation method of benzofuran compound
A compound and methyl technology, applied in the field of preparation of benzofuran compounds, can solve the problems of harsh conditions, complex preparation methods, poor environmental protection and the like, and achieve the effects of low cost, simple and convenient operation, and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
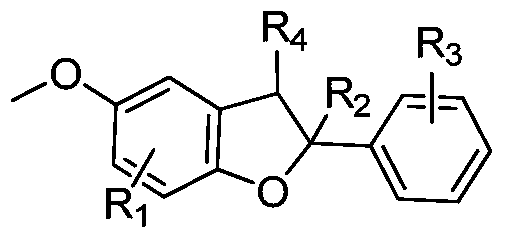
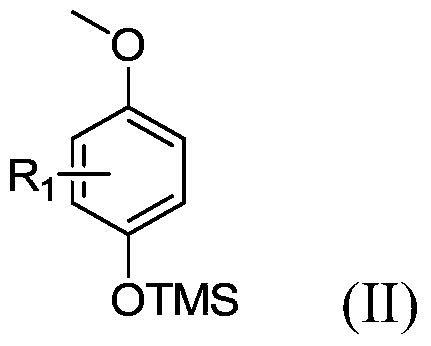
[0032] According to one aspect of the present invention, there is provided a method for preparing benzofuran compounds, the method comprising: under a nitrogen atmosphere, in the presence of an organic solvent, using persulfate as an oxidant, trimethylsilyl group-protected P-methoxyphenol compounds and aromatic olefin compounds are stirred and reacted under heating conditions, and after reacting for a certain period of time, benzofuran compounds are obtained through separation and purification.
[0033] In the present invention, examples of the "halogen" include F, Cl, Br, I, etc., preferably F, Cl, Br.
Embodiment 1
[0039] Add 40mg 4-methoxyphenoxytrimethylsilane, 145mg sodium persulfate and 85mg styrene to a 10mL round bottom flask, seal the flask and replace nitrogen 3 times, add 2ml of acetonitrile to the reaction flask with a needle, at 50 Reaction at ℃ for 72h. After the reaction, the reaction solution was filtered, concentrated and spin-dried, and then the mixed solution of petroleum ether: ethyl acetate with a volume ratio of 30:1 was used as an eluent to perform purification and separation by silica gel column chromatography to obtain 2-phenyl -5-methoxy-2,3-dihydrobenzofuran, whose structural formula is:
[0040]
[0041] The obtained 2-phenyl-5-methoxy-2,3-dihydrobenzofuran was a colorless liquid with a yield of 72%, and its NMR data was: 1 H NMR (400MHz, CDCl 3 )δ7.42–7.30(m,5H),6.83–6.73(m,2H),6.70(dd,J=8.6,2.6Hz,1H),5.74(t,J=8.8Hz,1H),3.77(s , 3H), 3.60 (dd, J=15.7, 9.3Hz, 1H), 3.20 (dd, J=15.7, 8.2Hz, 1H).
[0042] 13 C NMR (100MHz, CDCl 3 )δ 154.33, 153.81, 142.04,...
Embodiment 2
[0044] Add 40mg 4-methoxyphenoxytrimethylsilane, 97mg sodium persulfate and 85mg styrene to a 10mL round-bottomed flask, seal the flask and replace nitrogen 3 times, add 2ml acetonitrile to the reaction flask with a needle, at 50 Reaction at ℃ for 72h. After the reaction, the reaction solution was filtered, concentrated and spin-dried, and then the mixed solution of petroleum ether: ethyl acetate with a volume ratio of 30:1 was used as the eluent to perform purification and separation by silica gel column chromatography to obtain 2-phenyl -5-methoxy-2,3-dihydrobenzofuran, whose structural formula is:
[0045]
[0046] The obtained 2-phenyl-5-methoxy-2,3-dihydrobenzofuran was a colorless liquid with a yield of 29%, and its NMR data was: 1 H NMR (400MHz, CDCl 3 )δ7.42–7.30(m,5H),6.83–6.73(m,2H),6.70(dd,J=8.6,2.6Hz,1H),5.74(t,J=8.8Hz,1H),3.77(s , 3H), 3.60 (dd, J=15.7, 9.3Hz, 1H), 3.20 (dd, J=15.7, 8.2Hz, 1H).
[0047] 13 C NMR (100MHz, CDCl 3 )δ 154.33, 153.81, 142.04, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com