Synthesis method of brominated phenolic compound
A synthesis method and compound technology, applied in the field of synthesis of brominated phenolic compounds, can solve the problems of low reaction yield and poor environmental protection, and achieve the effects of low reaction cost, wide application range and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
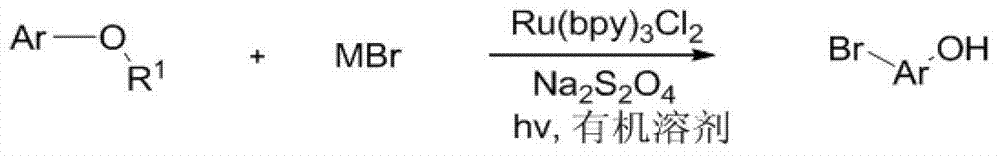
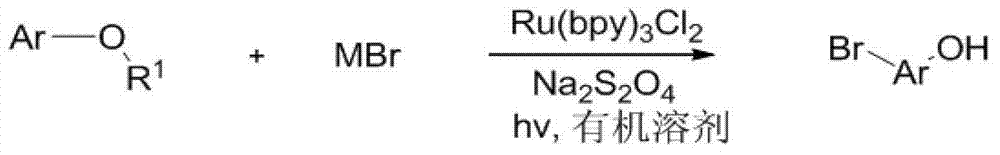
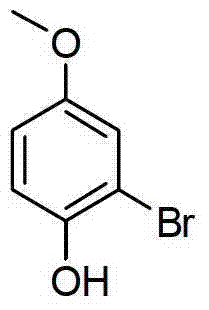
[0015] Specific embodiment one: the synthetic method of brominated phenolic compound of the present embodiment is that phenol derivatives, terpyridine ruthenium chloride, sodium persulfate, metal bromide and organic solvent are joined in the reaction vessel, as under visible light irradiation condition The stirring reaction at room temperature obtains the reaction solution, and the reaction solution is separated and purified through aftertreatment, and the synthesis of the brominated phenolic compound is completed, and the general reaction formula is as follows:
[0016]
[0017] Where Ar represents a non-heterocyclic aromatic group or a non-heterocyclic aromatic group with substituents, R 1 stands for hydrogen, alkyl, acyl, alkoxy, sulfonyl or silyl, MBr stands for metal bromide, and hv stands for visible light produced by incandescent lamps, LED lamps or the sun.
[0018] The raw materials metal bromide, terpyridyl ruthenium chloride, sodium persulfate and organic solvent...
specific Embodiment approach 2
[0025] Embodiment 2: This embodiment is different from Embodiment 1 in that the non-heterocyclic aromatic group is phenyl or naphthyl. Other steps and parameters are the same as those in Embodiment 1.
specific Embodiment approach 3
[0026] Specific embodiment three: this embodiment is different from specific embodiment one or two in that Ar represents a non-heterocyclic aromatic group with substituents, wherein the substituents are one or more of alkyl, alkoxy, and halogen kind. Other steps and parameters are the same as those in Embodiment 1 or Embodiment 2.
[0027] In this embodiment, Ar represents a non-heterocyclic aromatic group with a substituent, and the substituent can be one or more identical or different substituents. When there are multiple substituents, the adjacent two substituents are independent of each other or into a ring.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com