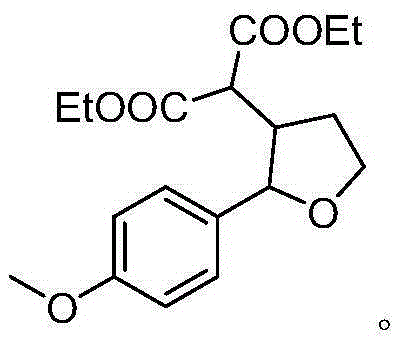
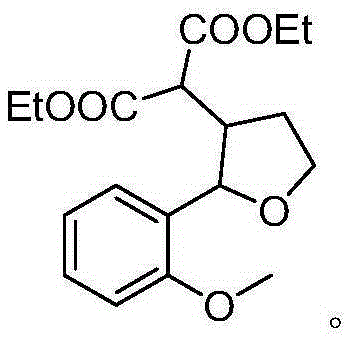
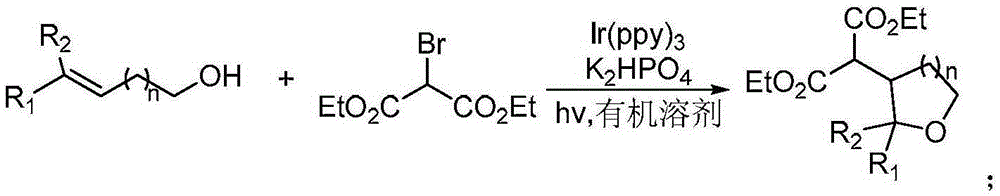A kind of synthetic method of β-diethyl malonate substituted tetrahydrofuran compound
A technology of diethyl malonate and tetrahydrofuran, which is applied in the synthesis field of replacing tetrahydrofuran compounds, can solve the problems of environmental pollution and low yield, and achieve the effects of simple and convenient operation, high yield and high economic benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0008] Specific embodiment one: this embodiment is a synthetic method of β-diethyl malonate substituted tetrahydrofuran compounds, which is specifically completed according to the following steps:
[0009] Add enol compounds, oxidizing reagents, photoreaction catalysts, dipotassium hydrogen phosphate and dry organic solvents into the reactor, and react at room temperature for 3 to 8 hours under visible light irradiation. After the reaction is complete, add distilled water into the reaction system to obtain The mixture is then extracted with ethyl acetate as an extractant to obtain an organic phase, which is dried over anhydrous sodium sulfate and concentrated, and then purified by silica gel column chromatography with an eluent to obtain β-diethyl malonate Substituted tetrahydrofuran compounds; the molar ratio of the enol compound to the oxidizing agent is 1:(1.2~2); the molar ratio of the enol compound to the photoreaction catalyst is 1:(0.01~0.05); The mol ratio of described...
specific Embodiment approach 2
[0015] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the structural formula of the enol compound is n is 1 or 2; the R 1 It is a non-heterocyclic aromatic group with an electron-donating substituent, wherein the electron-donating substituent is methyl, alkoxy or siloxy; the non-heterocyclic aromatic group is phenyl or naphthyl; the R 2 It is hydrogen, methyl, non-heterocyclic aromatic group or non-heterocyclic aromatic group with substituents, wherein the substituents are electron-withdrawing or electron-donating groups; non-heterocyclic aromatic groups are phenyl or naphthyl . Other steps are the same as in the first embodiment.
[0016] The electron-withdrawing or electron-donating groups described in this embodiment, such as halogen or alkoxy.
specific Embodiment approach 3
[0017] Embodiment 3: This embodiment differs from Embodiment 1 or Embodiment 2 in that: the oxidizing agent is diethyl bromomalonate. Other steps are the same as those in Embodiment 1 or 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com