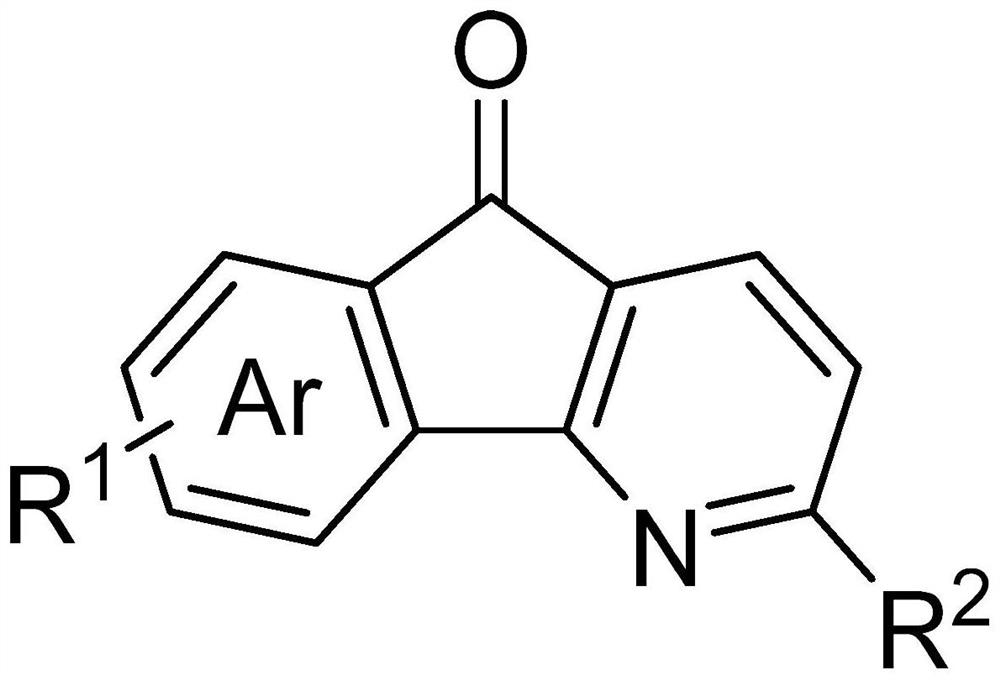
A kind of azafluorenone derivatives and preparation method and application thereof
A technology for azafluorenone and derivatives is applied in the field of azafluorenone derivatives and their preparation, which can solve the problems of harsh reaction conditions, cumbersome steps, need to be developed and the like, and achieves the effect of increasing complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
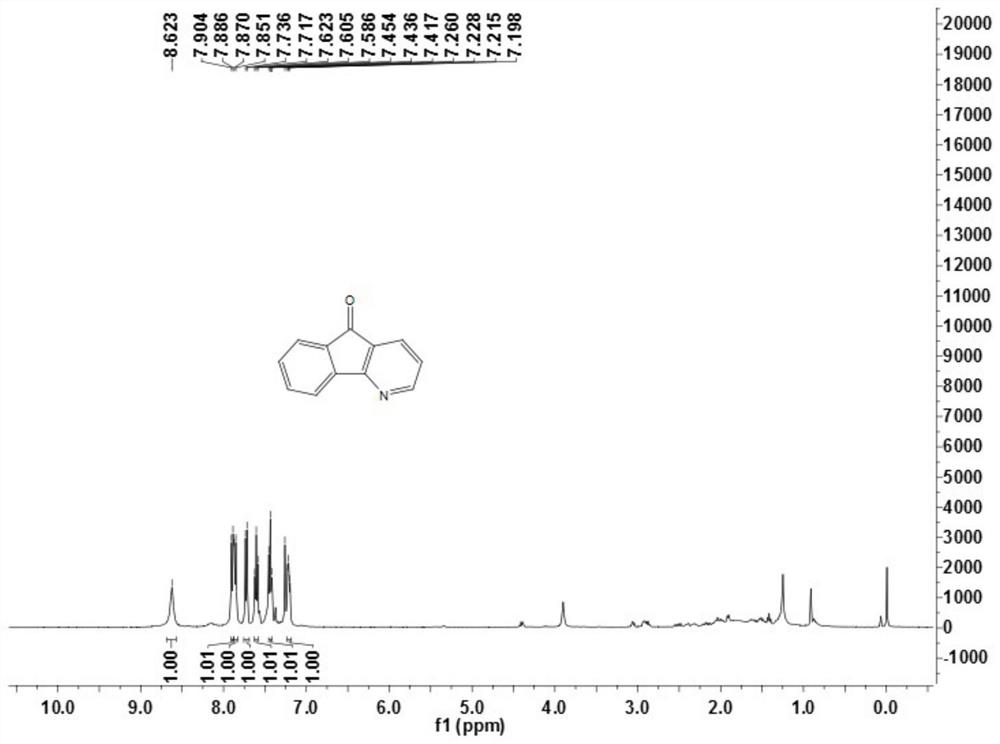
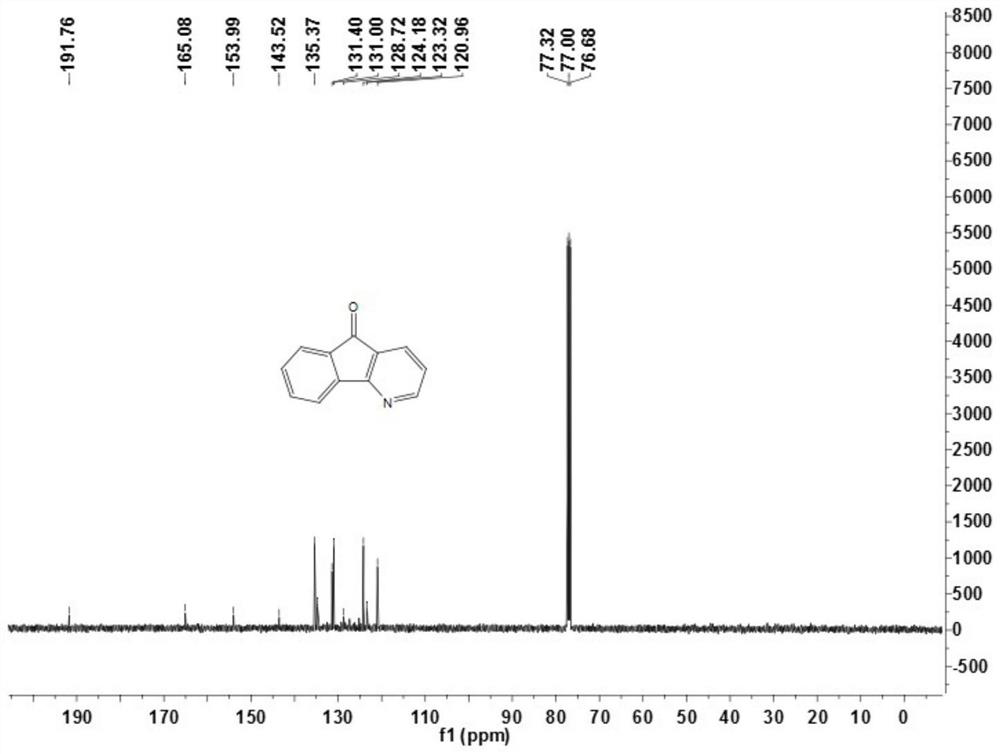
[0070] This embodiment provides compound 1a, its preparation method is as follows:
[0071]5H-indeno[1,2-b]pyridin-5-one (1a), the reaction formula is as follows:
[0072]
[0073] Under air atmosphere, the aryl imide ester compound 2a (30.0mg, 0.2mmol) shown in formula (II) was sequentially added into the reactor, and the R of formula (II) 1 is hydrogen, a solution of olefin compound 3a (28.0mg, 0.4mmol) shown in formula (III) in 1,2-dichloroethane (0.5mL), R in formula (III) is Me, R' is hydrogen, Subsequently, 2.5 mg of dichloro(pentamethylcyclopentadienyl) rhodium dimer, 7.8 mg of bistrifluoromethanesulfonimide silver salt, 4.9 mg of sodium acetate and 12.0 mg of copper acetate were added successively. React for 30 minutes, then use a syringe to inject a solution of acetic acid (1.5mL) in terminal alkene 4a (40μL, 0.40mmol) into the reactor, R of formula (IV) 2 For hydrogen, the reaction temperature was raised to 120°C to continue the reaction for 24 hours. The end of...
Embodiment 2
[0077] This embodiment provides compound 1b, its preparation method is as follows:
[0078] The full name of 1b is 2-methyl-5H-indeno[1,2-b]pyridin-5-one (1b), and its reaction formula is as follows:
[0079]
[0080] Under air atmosphere, the aryl imide ester compound 2a (30.0mg, 0.2mmol) shown in formula (II) was sequentially added into the reactor, and the R of formula (II) 1 is hydrogen, a solution of olefin compound 3a (35.0mg, 0.5mmol) shown in formula (III) in 1,2-dichloroethane (0.5mL), R in formula (III) is Me, R' is hydrogen, Subsequently, 2.5 mg of dichloro(pentamethylcyclopentadienyl) rhodium dimer, 7.8 mg of bistrifluoromethanesulfonimide silver salt, 4.9 mg of sodium acetate and 12.0 mg of copper acetate were added successively. React for 30 minutes, then use a syringe to inject a solution of acetic acid (1.5mL) in terminal alkene 4b (40μL, 0.40mmol) into the reactor, R of formula (IV) 2 For Me, the reaction temperature was raised to 120°C to continue the re...
Embodiment 3
[0084] This embodiment provides compound 1c, its preparation method is as follows:
[0085] The full name of 1c is 7-iodo-5H-indeno[1,2-b]pyridin-5-one (1c), and its reaction formula is as follows:
[0086]
[0087] Under the air atmosphere, the aryl imide ester compound 2b (55.0mg, 0.2mmol) shown in formula (II) was sequentially added into the reactor, and the R of formula (II) 1 Halogen I, a solution of olefin compound 3a (28.0mg, 0.4mmol) represented by formula (III) in 1,2-dichloroethane (0.5mL), R in formula (III) is Me, R' is hydrogen , followed by adding 2.5 mg of dichloro(pentamethylcyclopentadienyl) rhodium dimer, 7.8 mg of bistrifluoromethanesulfonimide silver salt, 4.9 mg of sodium acetate and 12.0 mg of copper acetate, at 100 ° C The reaction was carried out for 30 minutes, and then a solution of acetic acid (1.5 mL) in terminal alkene 4a (40 μL, 0.40 mmol) was injected into the reactor with a syringe, and R of formula (IV) 2 For hydrogen, the reaction tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com