Novel amyloid beta oligomer specific binding molecule
An amyloid and binding molecule technology, applied in the direction of immunoglobulin, anti-animal/human immunoglobulin, specific peptide, etc., can solve the problems of product instability and aggregation, poor tissue penetration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0066] IgNARs / vNARs - Overview
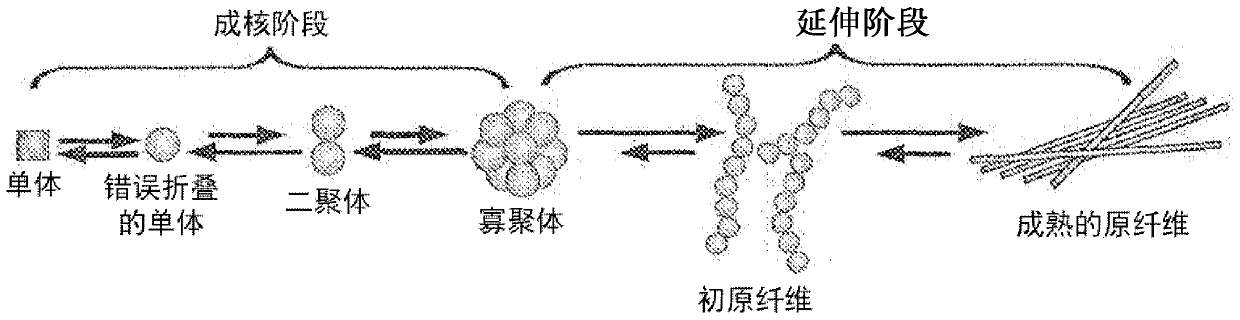
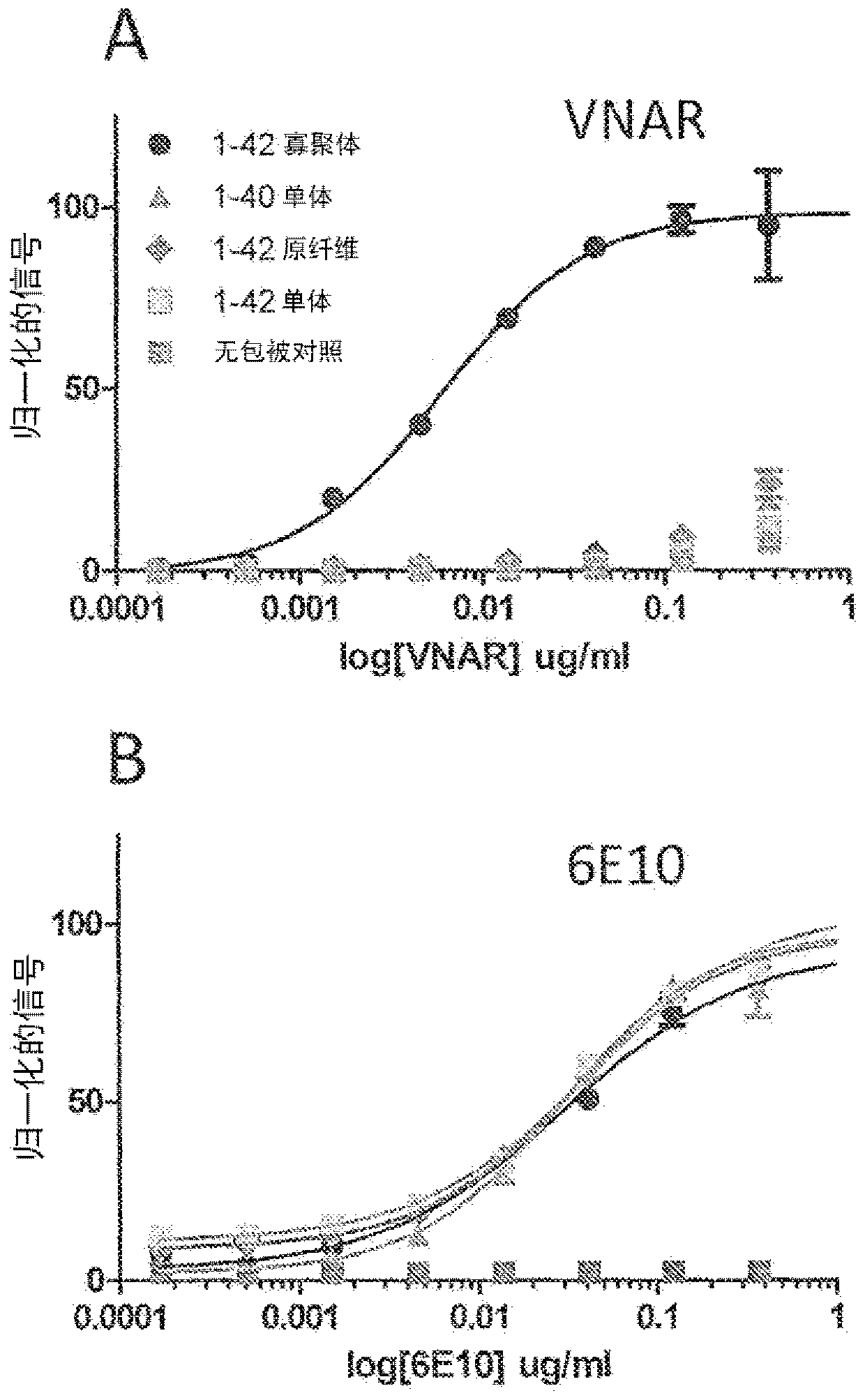
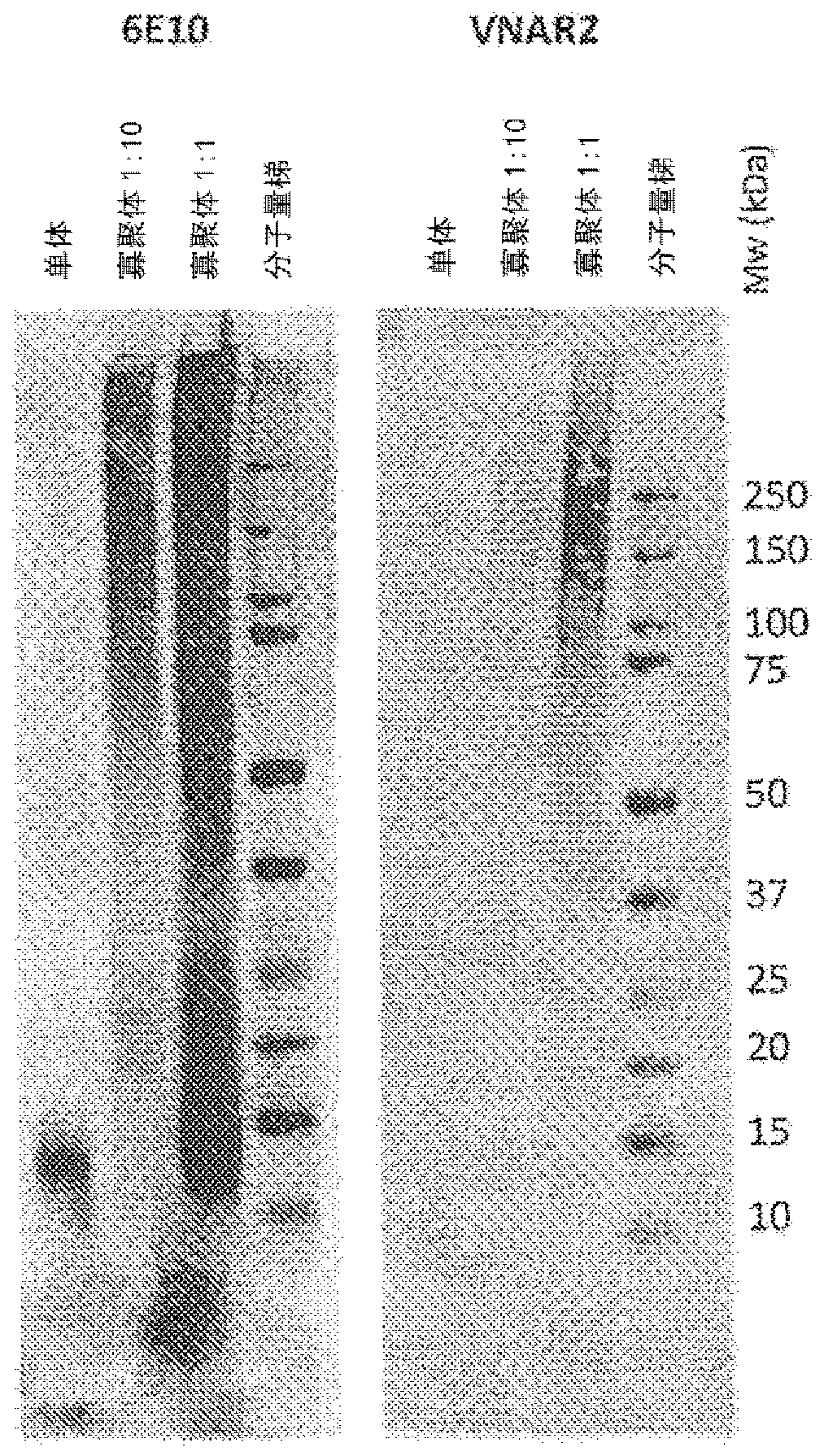
[0067] Cartilaginous fishes (sharks, rays, rays and chimaeras) express three distinct antibody isotypes: IgM, IgNAR (immunoglobulin neoantigen receptor) and primitive IgW (primordial IgW) (Rumfelt LL. et al. BMC immunology 2004 ; 5:8; Rumfelt LL et al. Journal of Immunology 2004; 173:1 129-39). IgNAR was first identified in the serum of the nurse shark (Ginglymostoma cirratum) (Greenberg AS et al. Nature 1995;374:168-73). IgNARs are heavy chain homodimers lacking light chains. Each chain in the secreted form consists of one variable region followed by five constant regions, the latter four being homologous to IgW constant regions. The antigen binding site is formed by only a single domain, called "vNAR" (variable region of neoantigen receptor). Serum IgNAR levels range from about 0.1 mg / ml to 1 mg / ml.
[0068] To date, all IgNARs identified have been reported to have a minimal variable loop region similar to the conventional CDR1 and CDR2 l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com