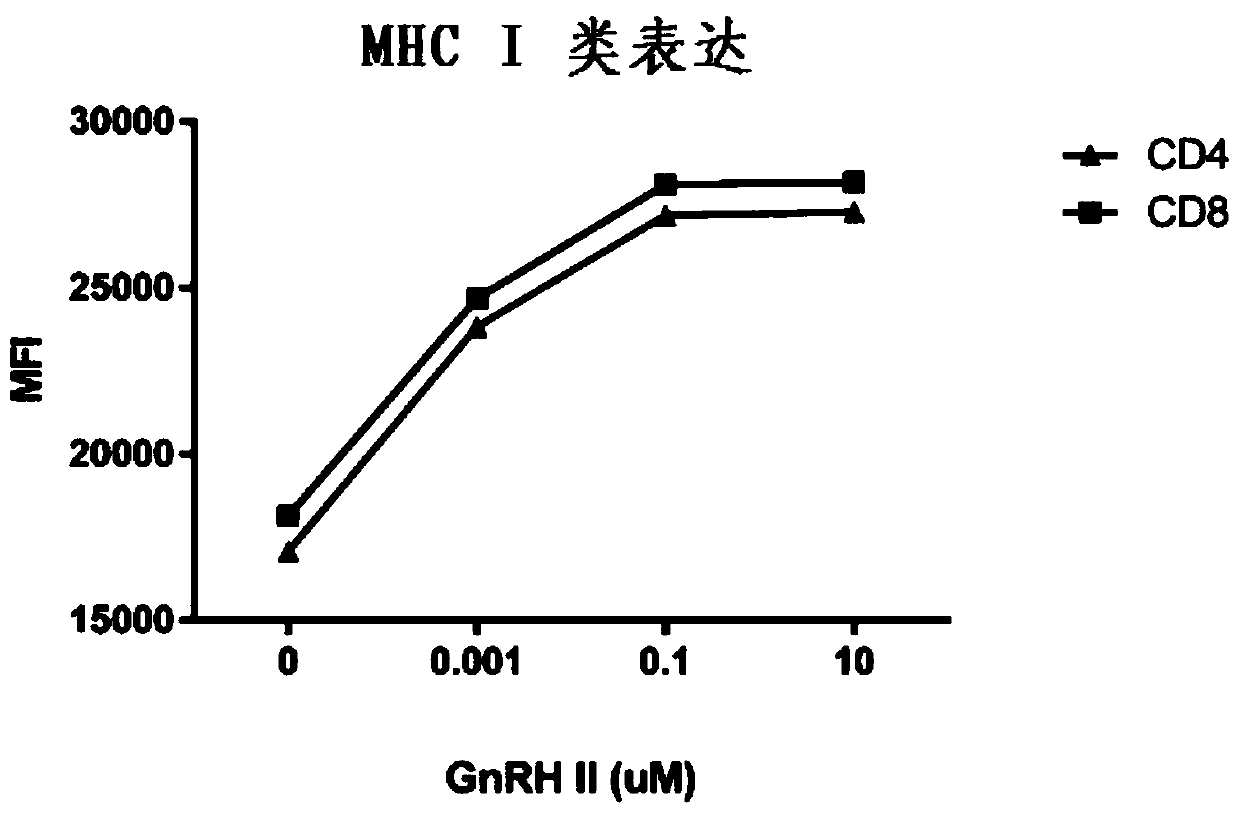
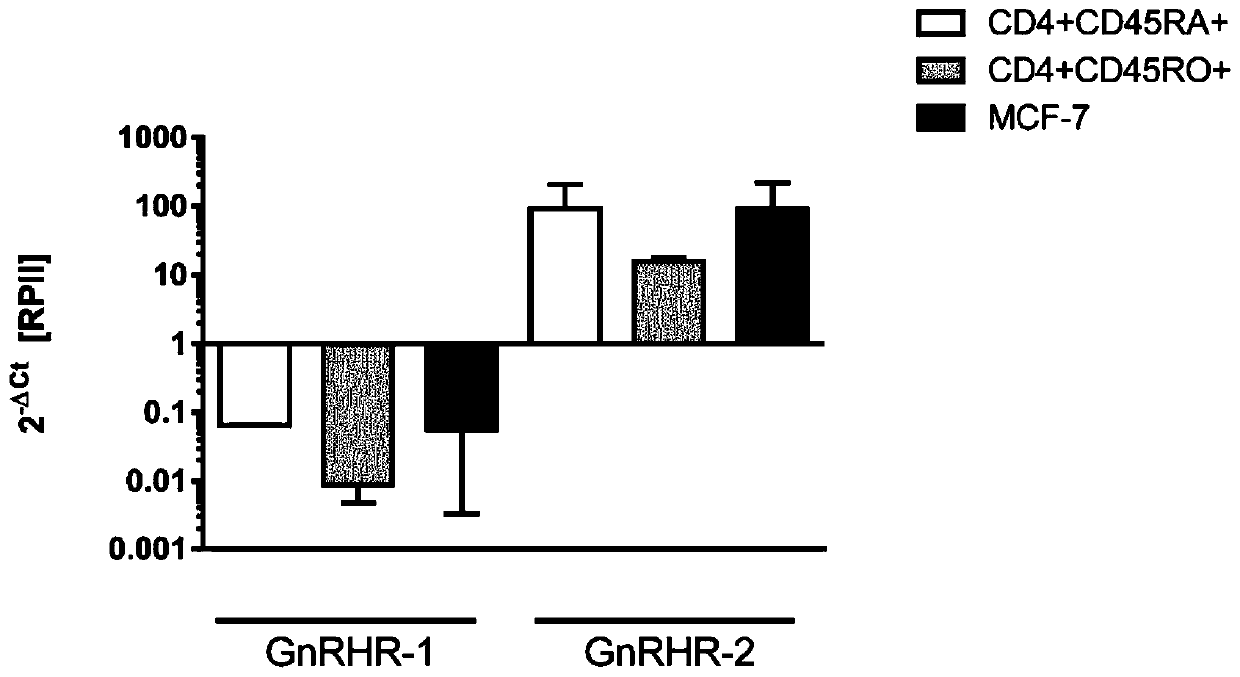
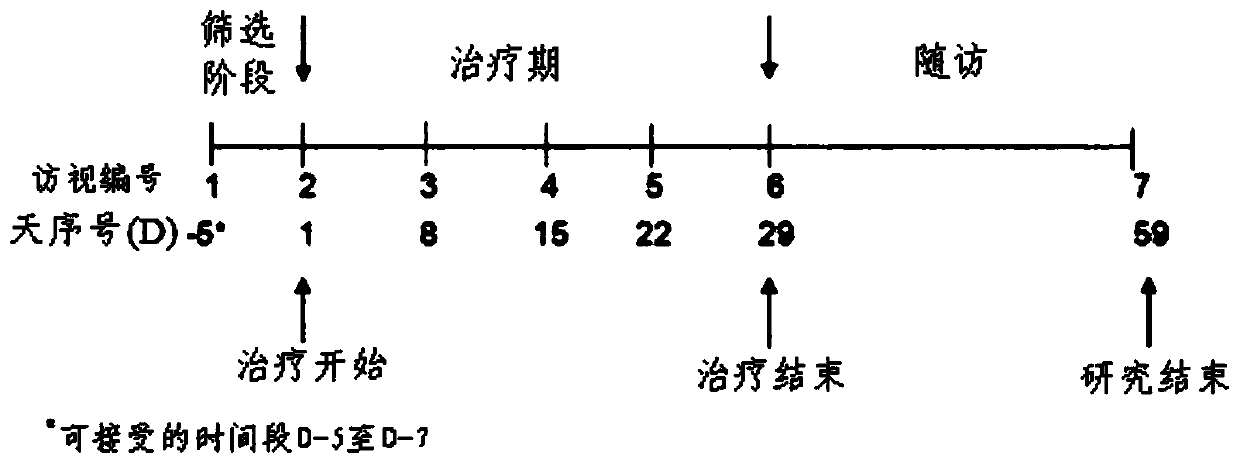
Gonadotropin-releasing hormones for use as adjuvant immunotherapeutics
An immunotherapy, sex hormone technology, applied in the direction of antiviral agents, allergic diseases, organic active ingredients, etc. Effects of long-term prevention of STD progression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Phase II clinical study of open label
[0077] An open-label, uncontrolled, single-center Phase IIa clinical study was conducted by the Clinical Research Group at the University of Pretoria, South Africa. Research outline such as image 3 shown. Subjects were HIV 1-infected antiretroviral therapy (ART)-naïve male patients (n=26). Inclusion criteria were asymptomatic, confirmed HIV-1 infection documented by HIV antibody test, male, age 18-50 years, CD4 + T cell count >350 cells / μl whole blood, and HLA-A*0205, HLA-A*3002, or HLA-B*1503 haplotype positive. The patient administered buserelin acetate nasal spray solution ( Sanofi-Aventis) 28 days. To compensate for any endocrine side effects, the patient received a further intramuscular injection of 150 mg testosterone cypionate ( - Testosterone, Pfizer). The primary study objective was to assess the safety and tolerability of intranasal buserelin acetate for 28 days, and the secondary study objectives w...
Embodiment 2
[0082] Example 2: Follow-up Study and Interim Analysis
[0083] To investigate the long-term effects of GnRH therapy in patients with HIV / AIDS, in particular to develop ways to minimize the risk of disease progression, a follow-up study and an interim analysis were performed 36-48 months after the open-label phase II clinical study . The aim of the study was to assess the progression of HIV infection over the time period from completion of the phase IIa clinical study (Example 1 ) based on assessment of past and present clinical, immunological and virological status.
[0084] Follow-up included 13 of the initial 26 patients, nine of whom underwent a full clinical examination. For interim analyses, disease progression was based on immunologic criteria and was defined as a medical condition in which a patient developed any AIDS-defining disease (eg, tuberculosis infection) during the period from the last day of treatment in the clinical trial to the day of follow-up, or CD4 me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com