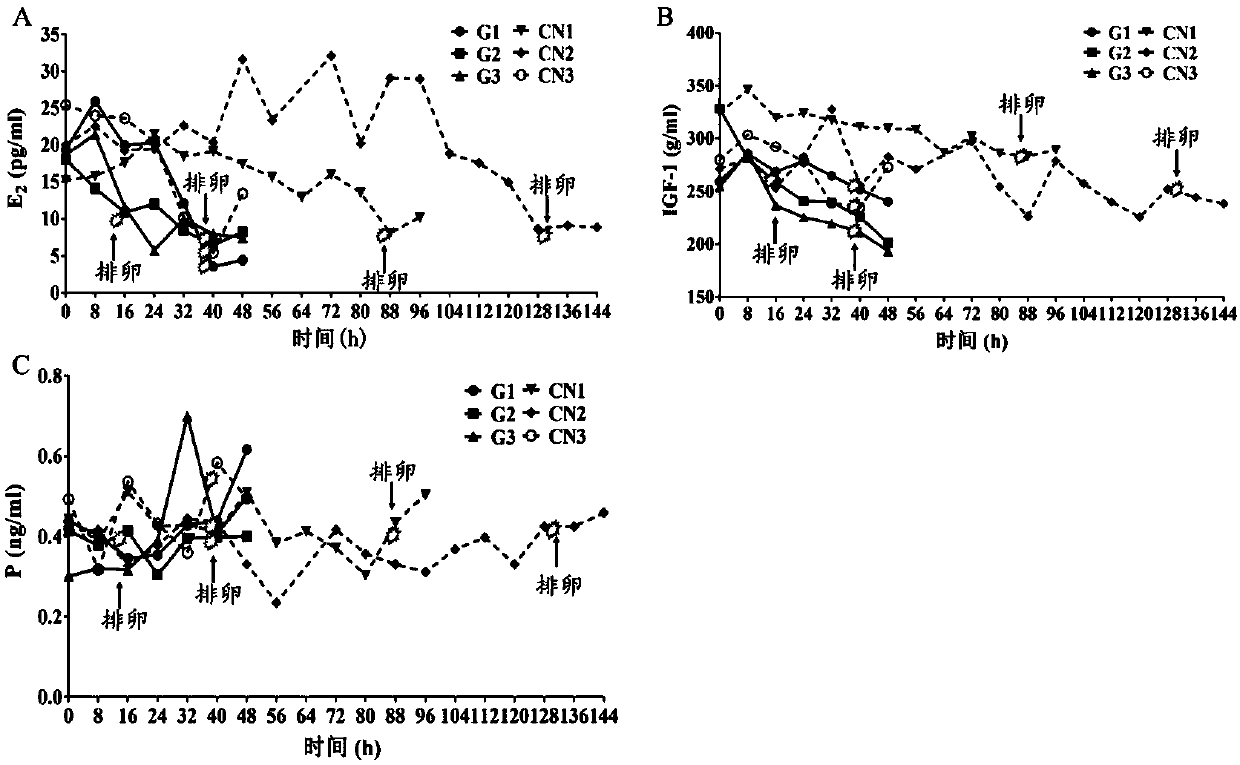
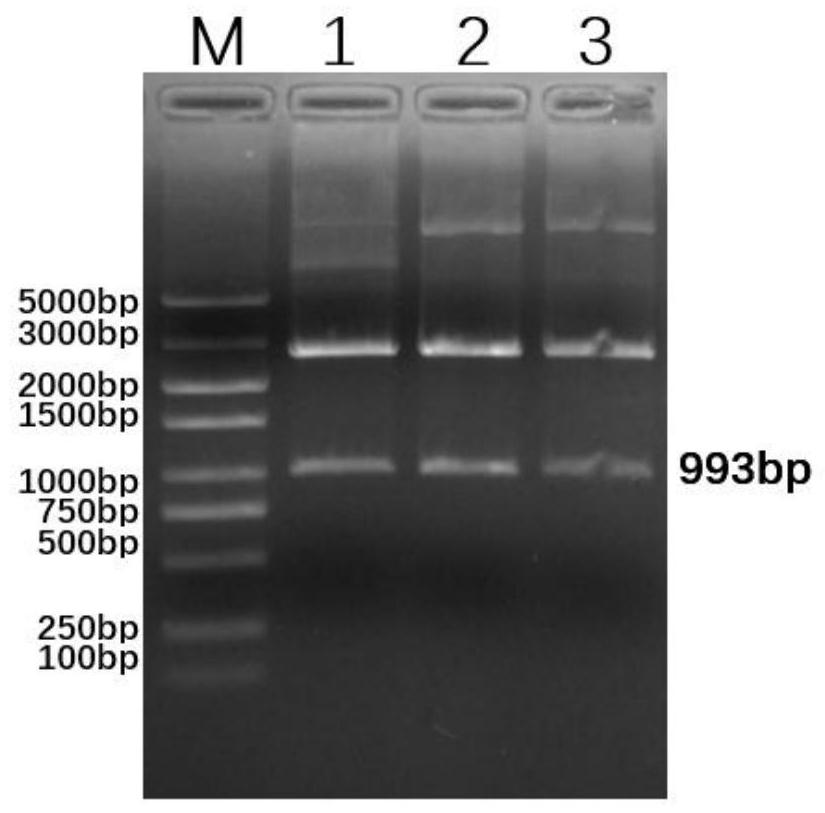
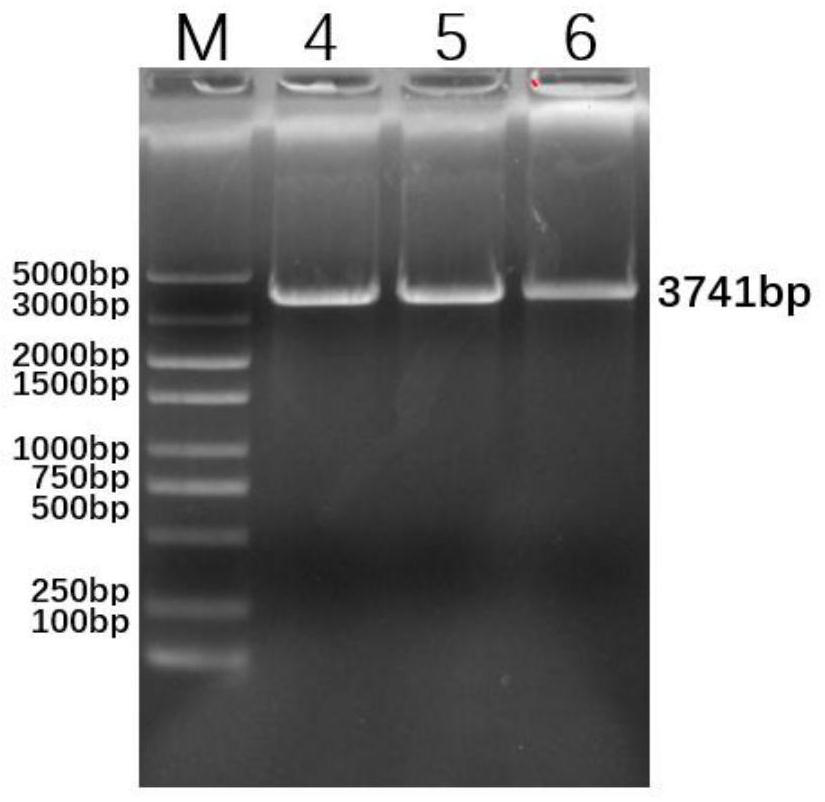
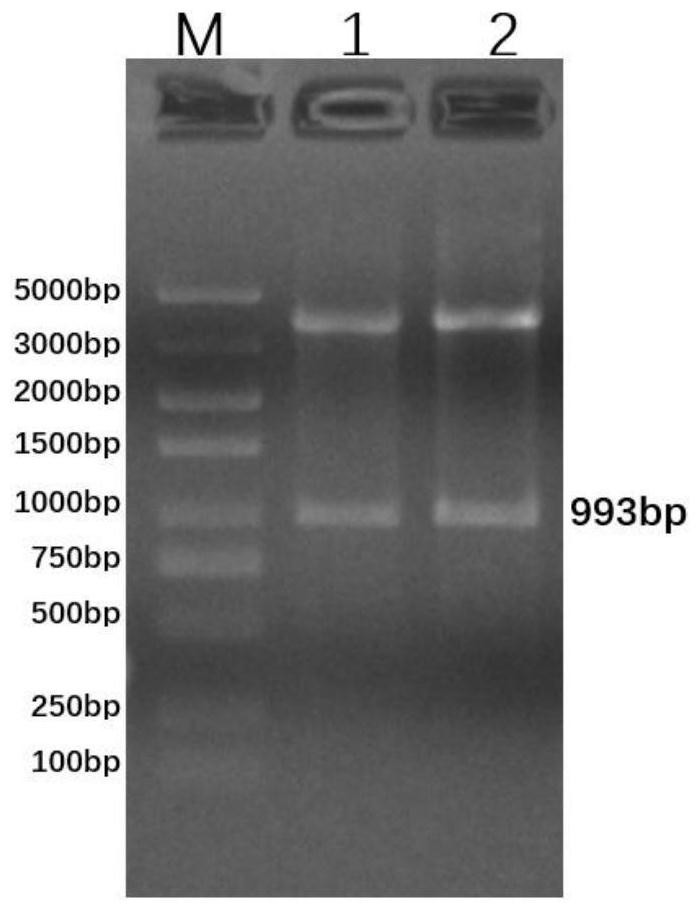
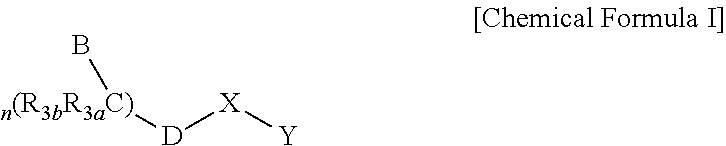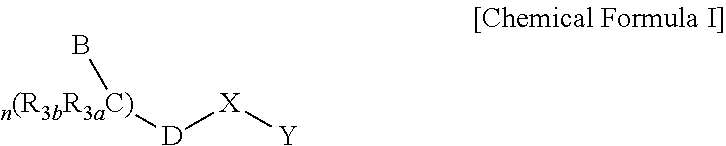Patents
Literature
41 results about "Gonadotrophin release hormone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
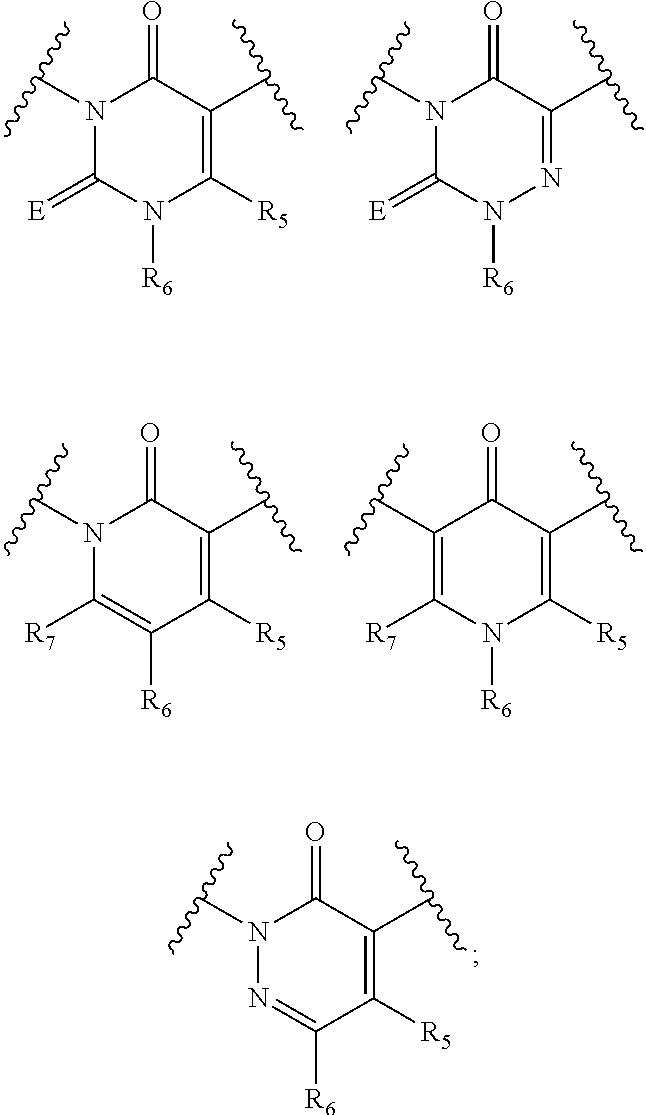
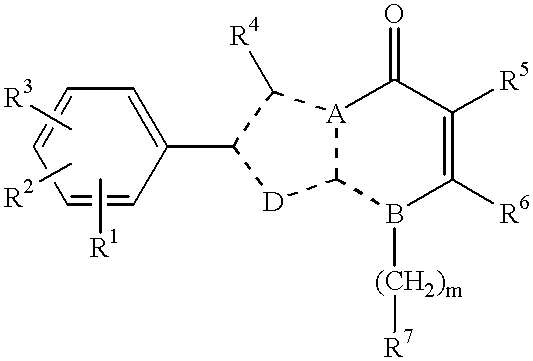
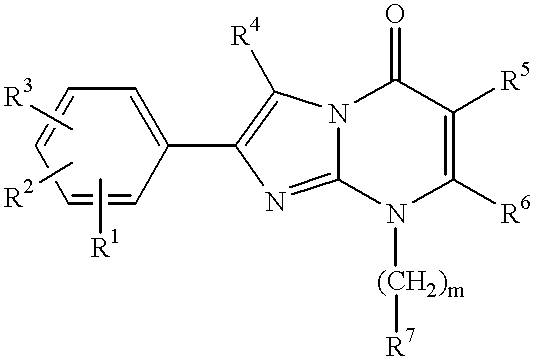
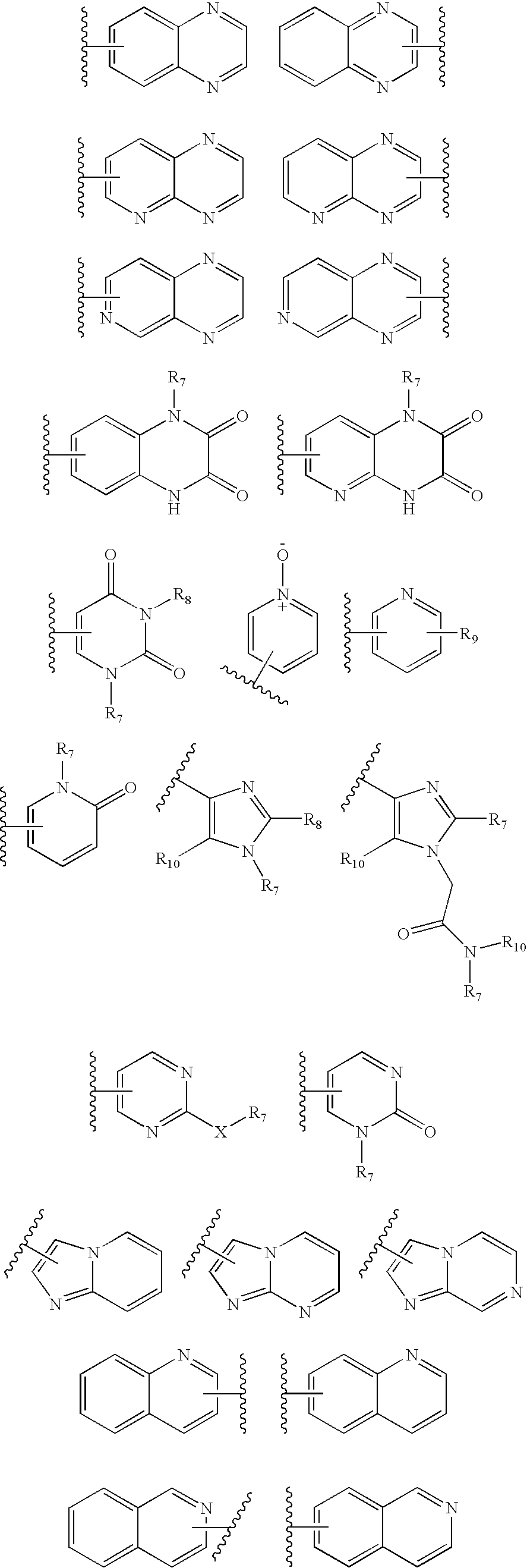
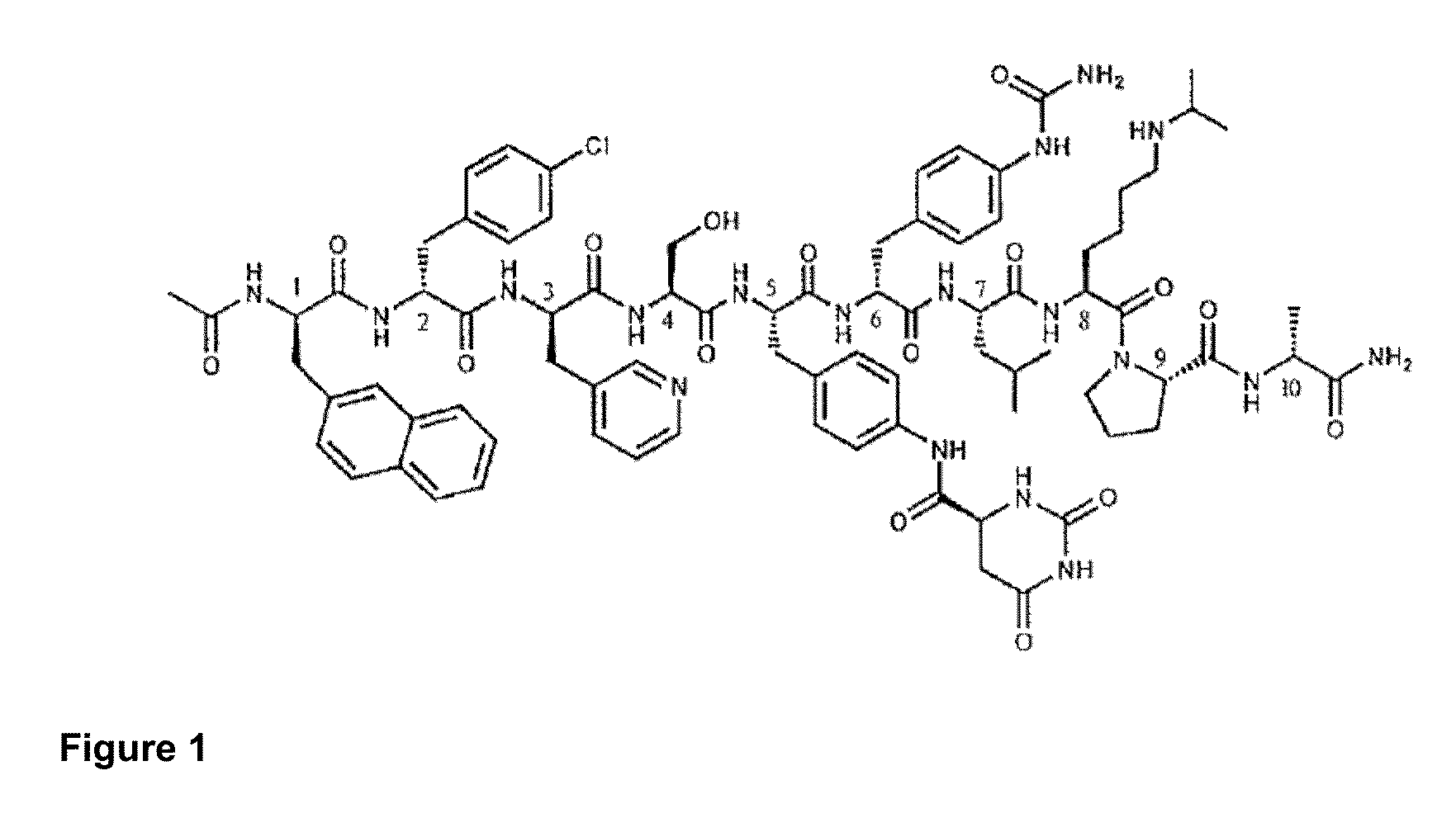
Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor
InactiveUS20060189617A1BiocideOrganic chemistryLutenizing hormoneGonadotropin-releasing hormone receptor
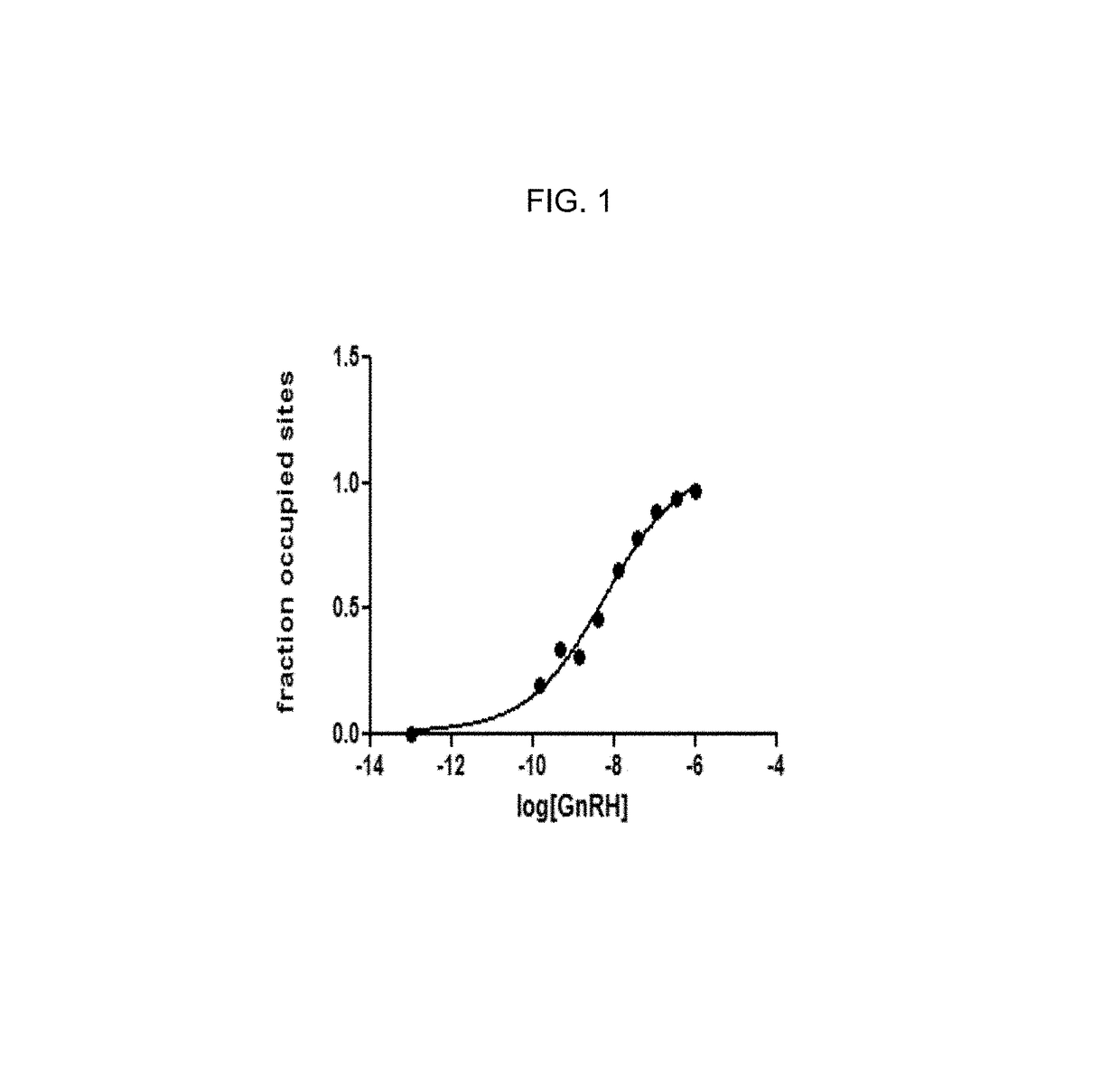
The present invention relates to Gonadotropin Releasing Hormone (GnRH, also known as Luteinizing Hormone Releasing Hormone) receptor antagonists.
Owner:WYETH
METHOD OF TREATING PROSTATE CANCER WITH GnRH ANTAGONIST
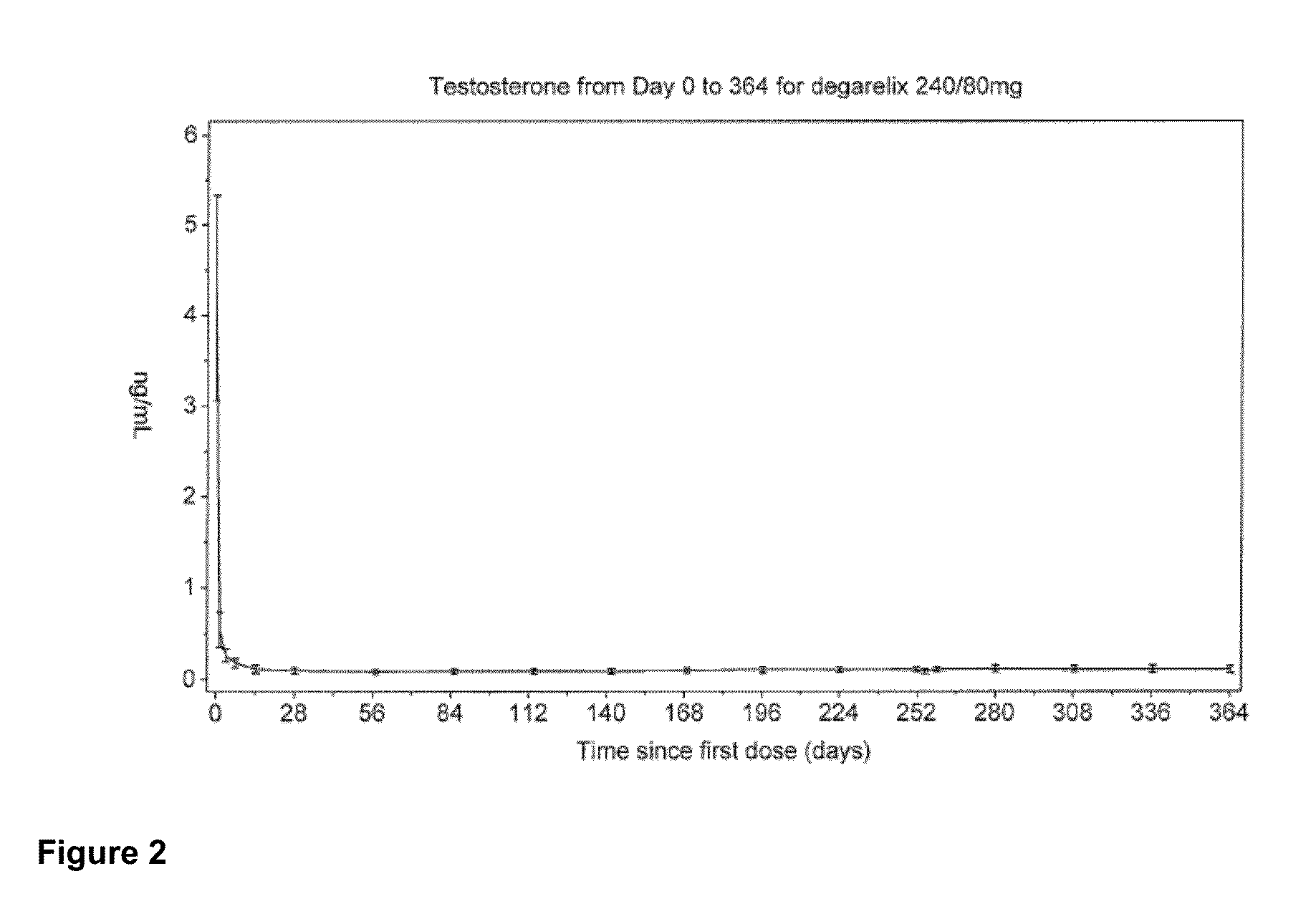
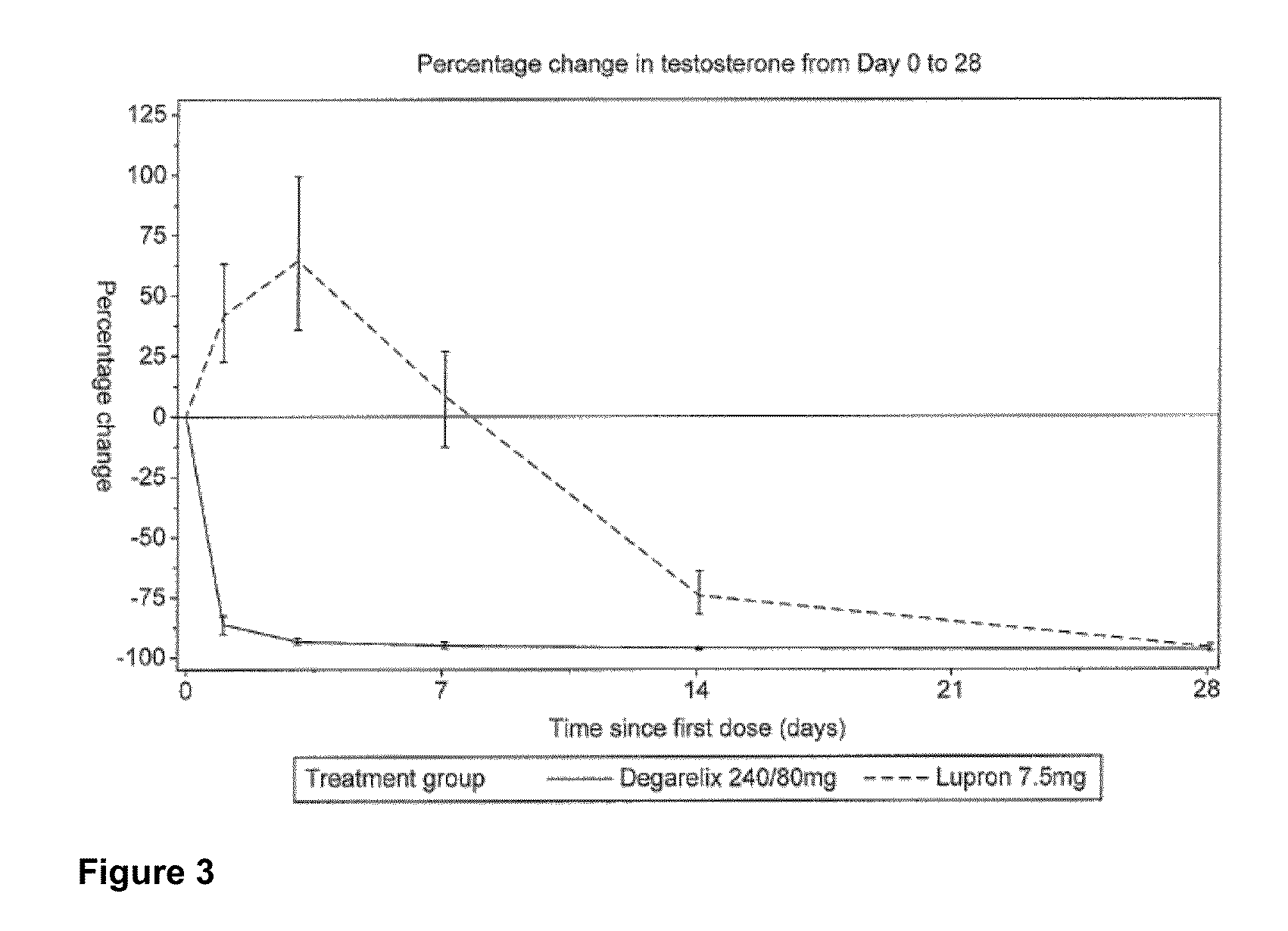
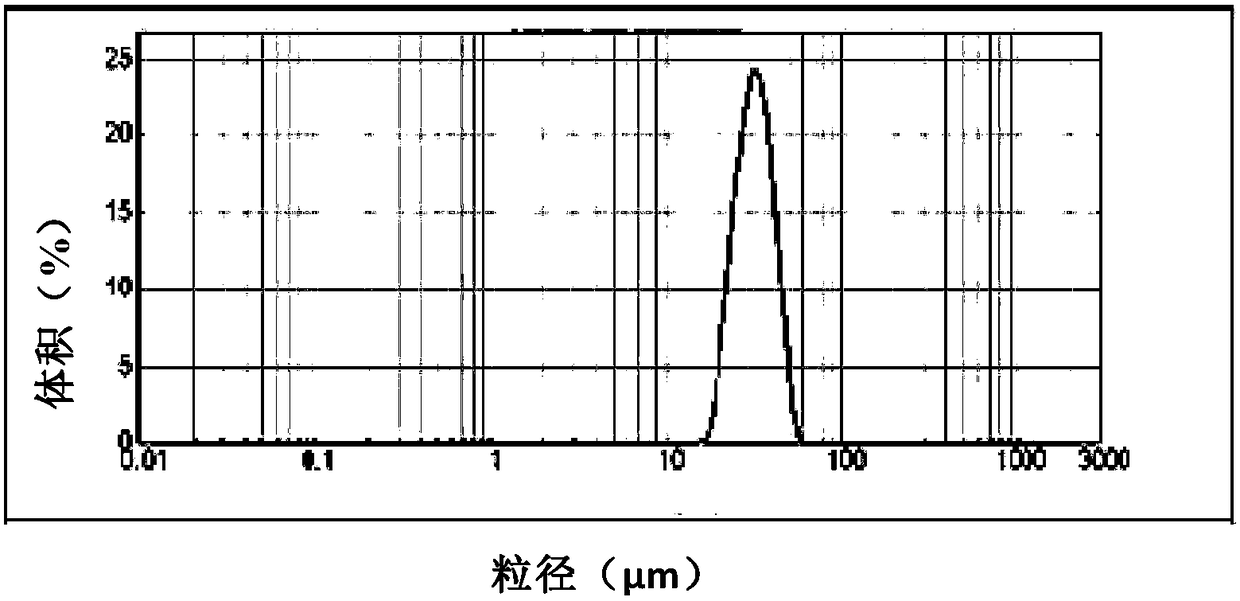
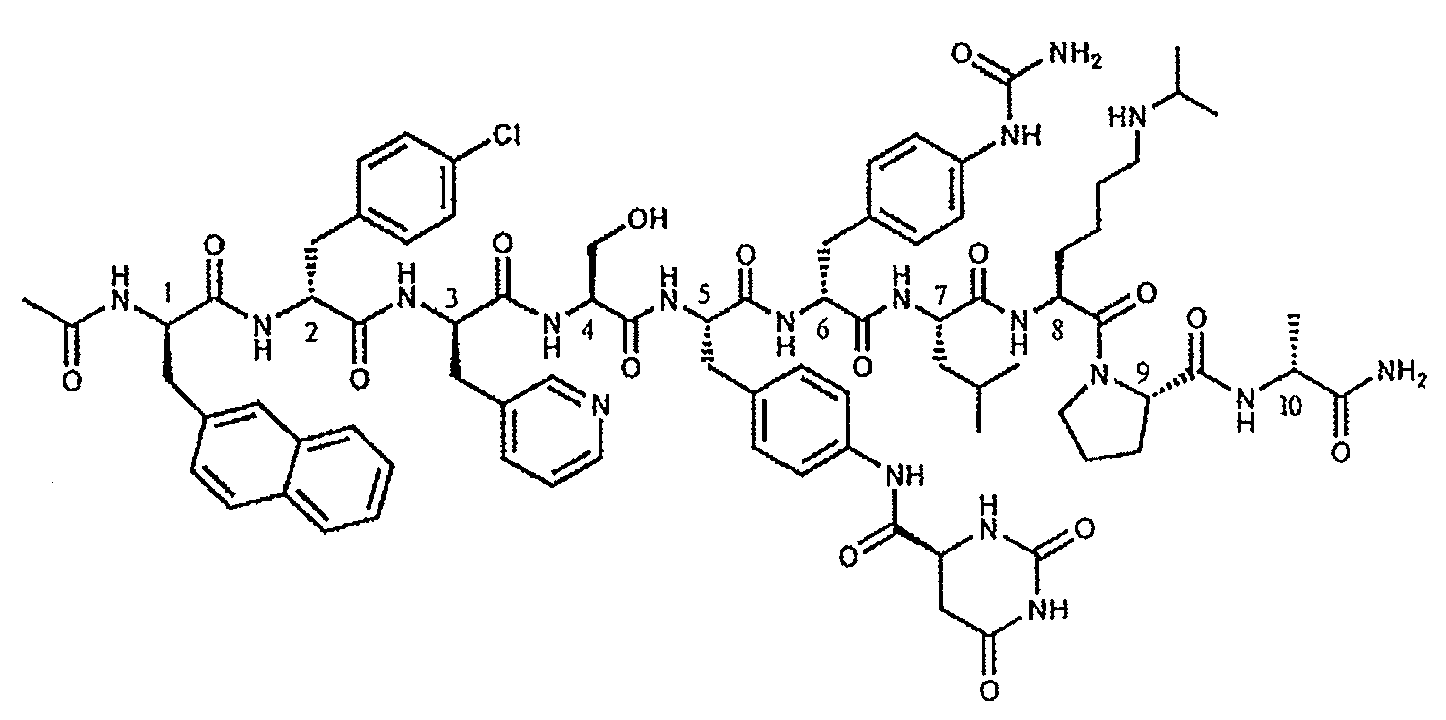
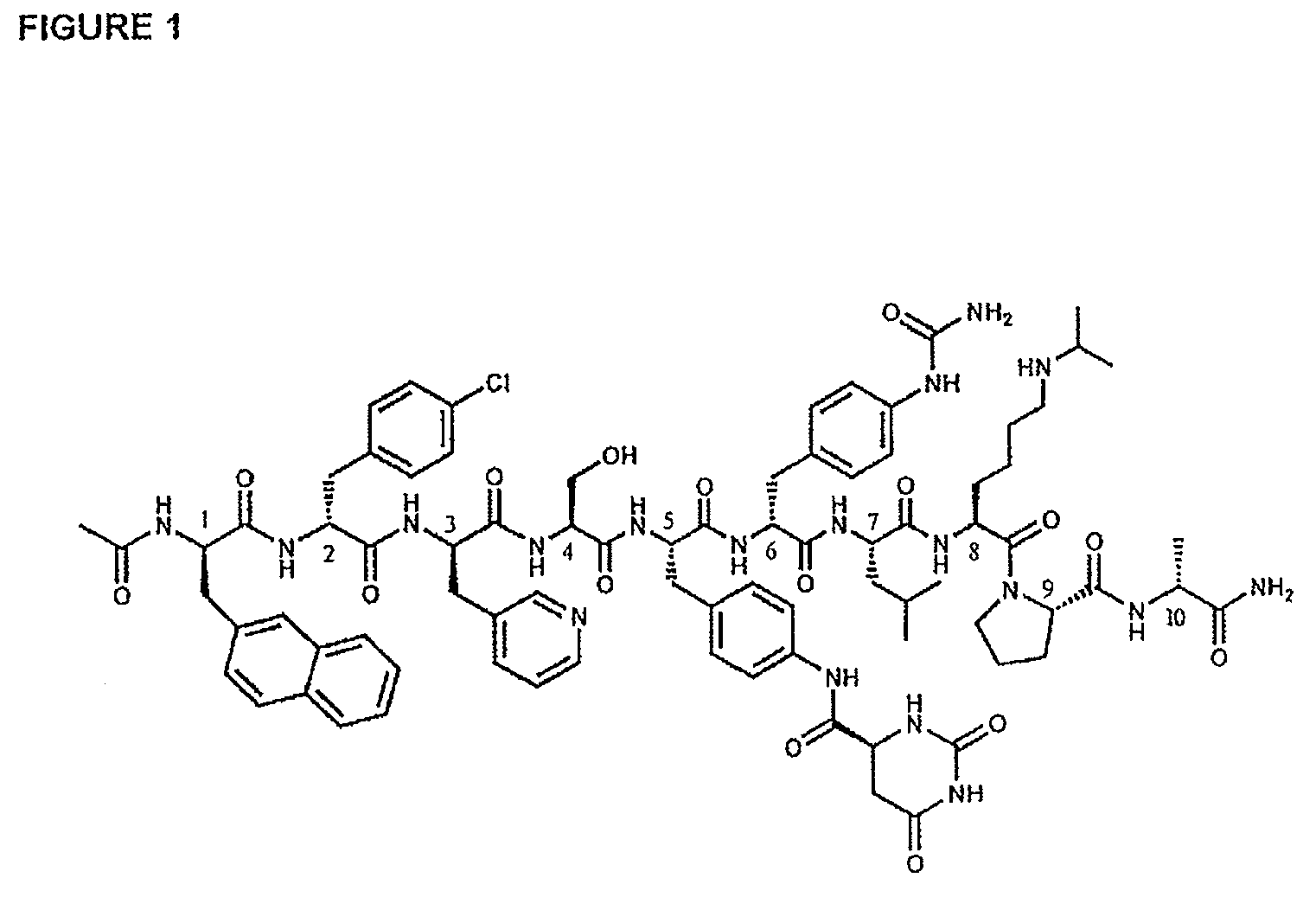
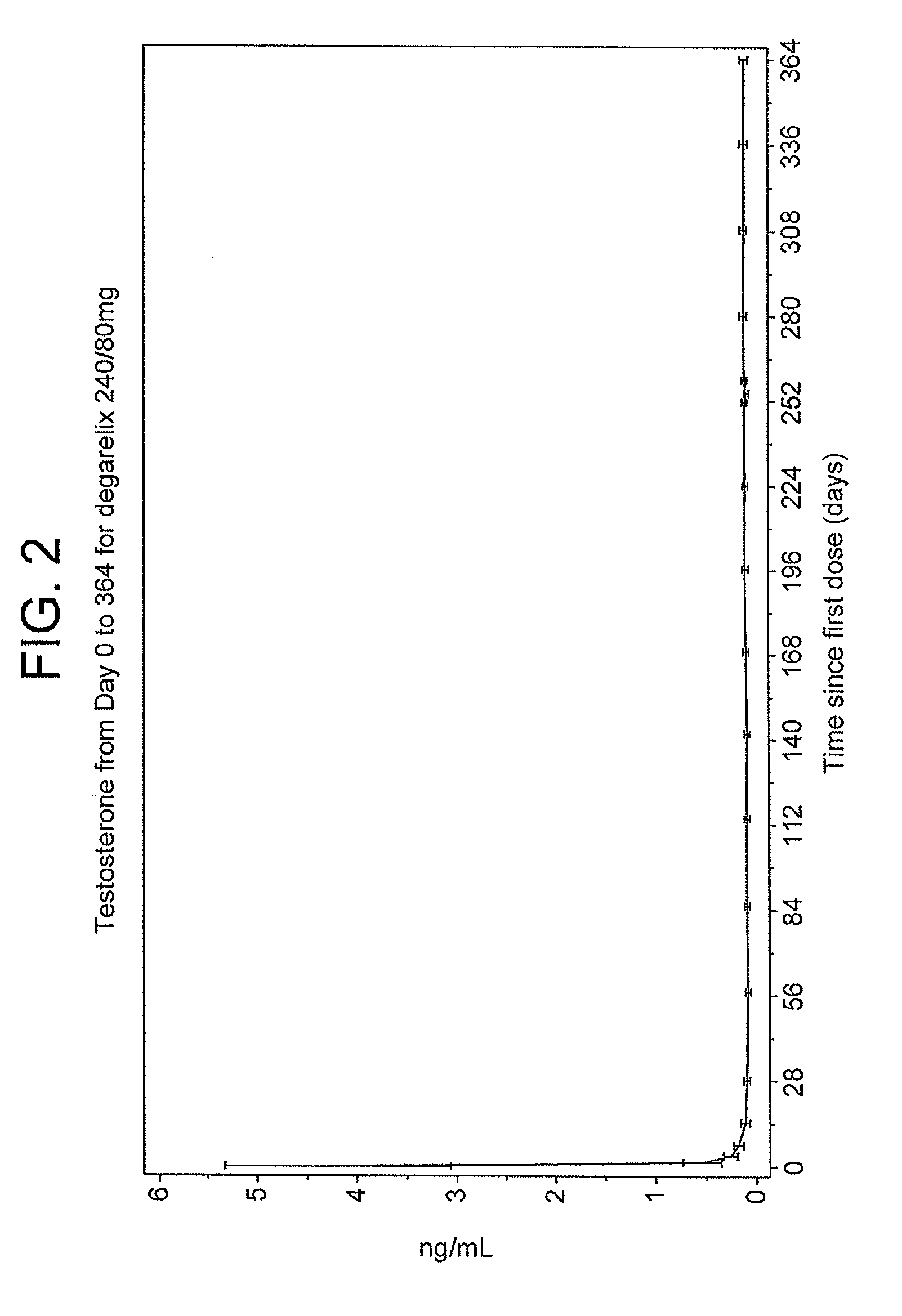
The invention provides methods and dosing regimens for safely and effectively treating androgen-dependent prostate cancer with a gonadotrophin releasing hormone (GnRH) antagonist without causing a testosterone spike and / or other side effect of GnRH agonist therapy such as a urinary tract infection, or an arthralgia-related or cardiovascular side effect. The present disclosure also provides for methods for treating prostate cancer in a patient with a history of at least one cardiovascular event, wherein administration of degarelix to the subject decreases the likelihood of developing or experiencing an additional cardiovascular event compared to treatment with a gonadotrophin releasing hormone (GnRH) agonist.
Owner:FERRING BV
Tetrahydrocarbazol derivatives as ligands for G-protein-coupled receptors (GPCR)
InactiveUS20030232873A1Low estrogen dosagesReduction in the hormone dosagesBiocidePeptide/protein ingredientsCarbazoleG protein-coupled receptor
This invention provides new tetrahydrocarbazole derivatives that act as ligands for G-protein-coupled receptors (GPCR), especially as antagonists of the gonadotropin-releasing hormone (GnRH). A pharmaceutical composition that contains these new tetrahydrocarbazole derivatives as well as a process for the production of the new tetrahydrocarbazole derivatives are also provided. Moreover, this invention relates to the administration of tetrahydrocarbazole derivatives for treating pathologic conditions that are mediated by GPCR, especially for inhibiting GnRH, in mammals, especially humans, who require such an administration, as well as the use of tetrahydrocarbazole derivatives for the production of a pharmaceutical agent for treating GPCR-mediated pathologic conditions, especially for inhibiting GnRH.
Owner:AETERNA ZENTARIS GMBH
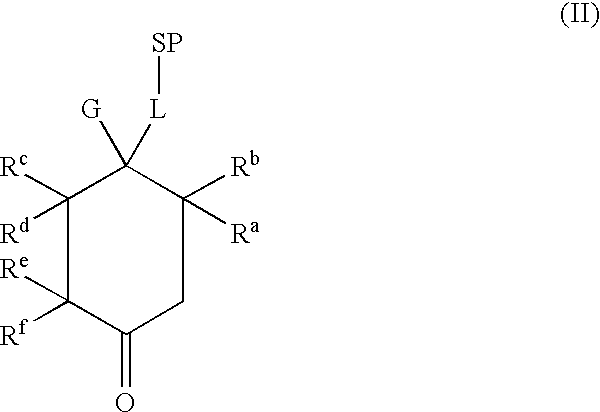
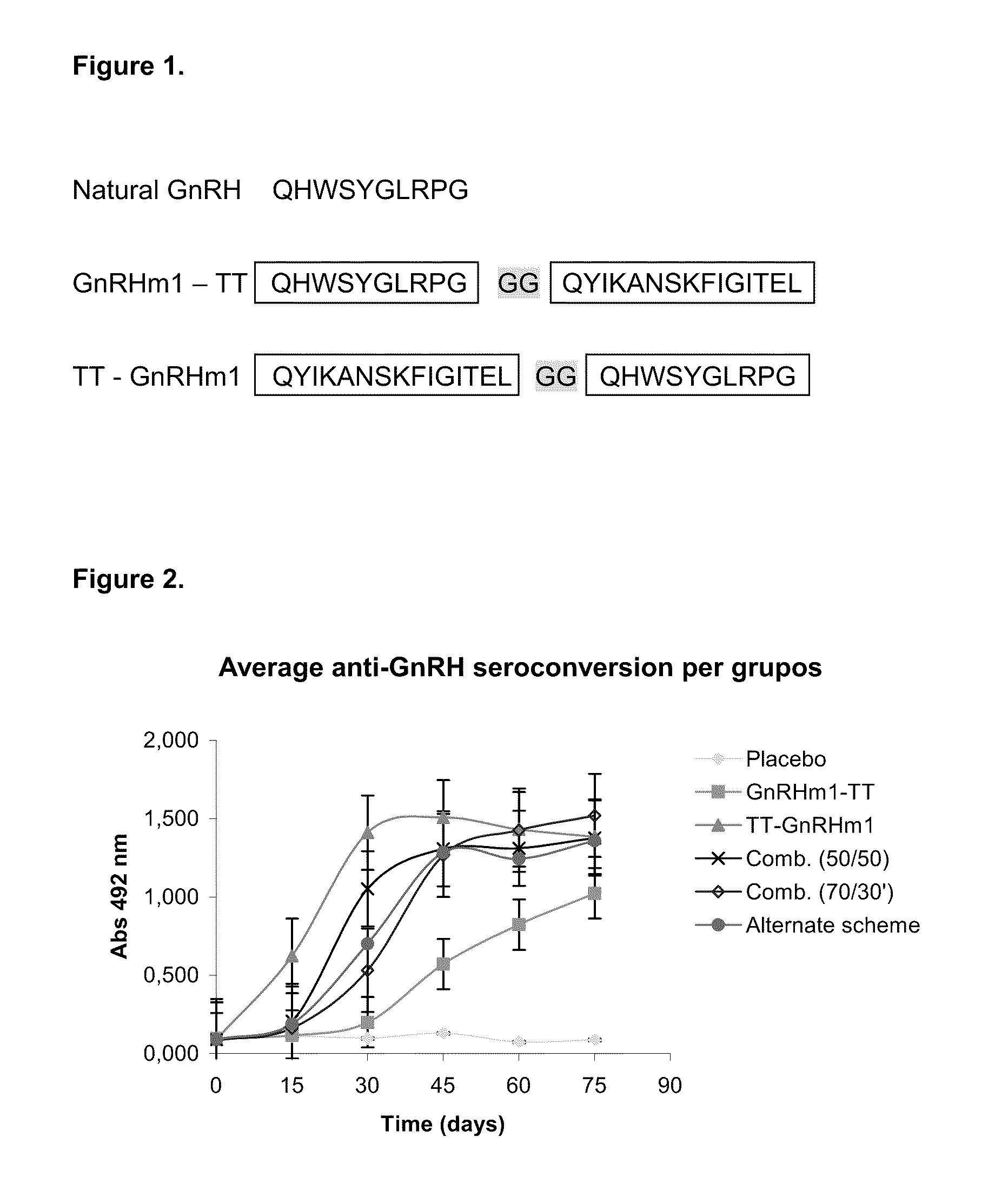
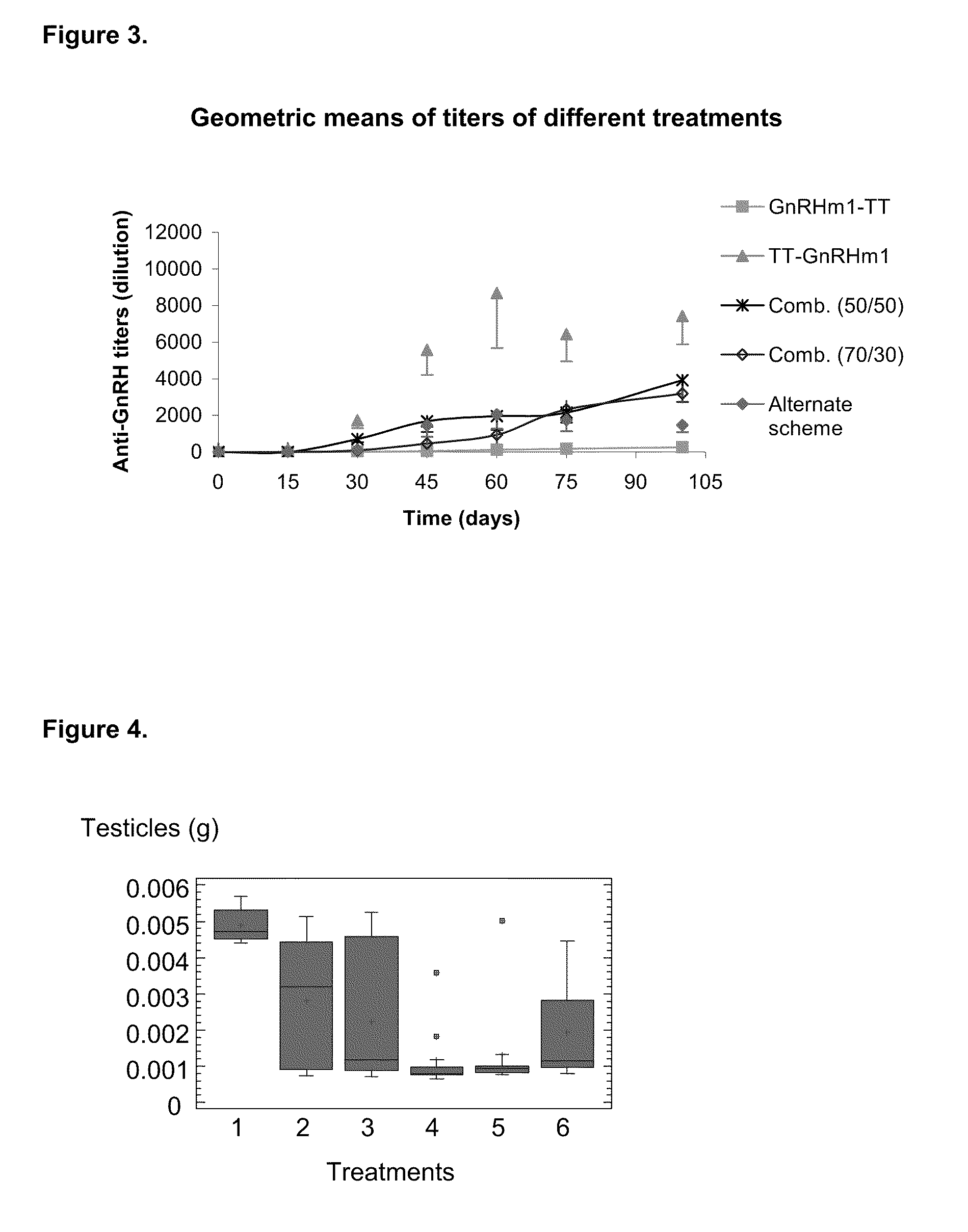
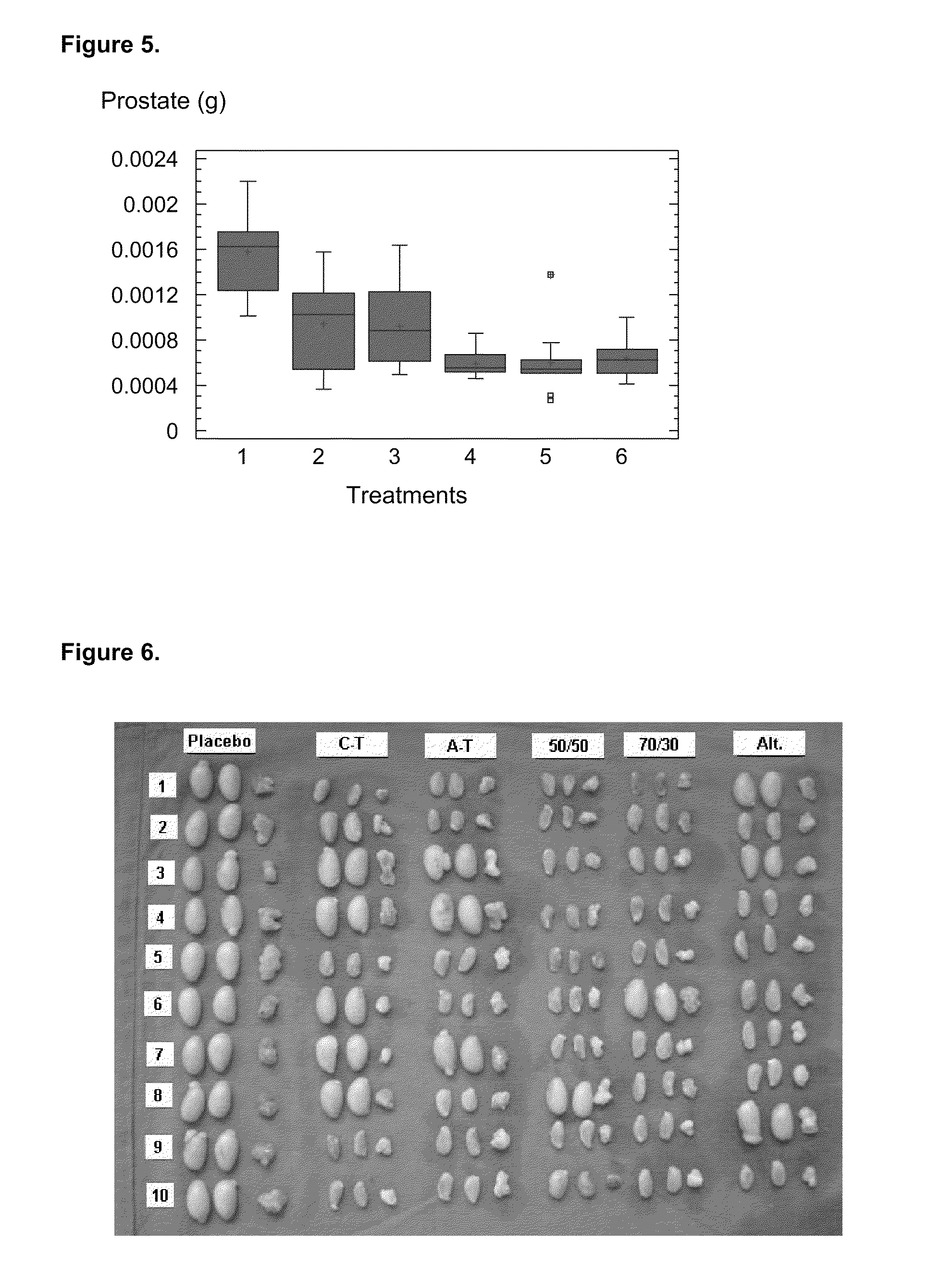
Pharmaceutical composition using gonadotropin - releasing hormone (GNRH) combined variants as immunogen
InactiveUS20110250196A1Faster and more potent immunological responseVigorous immunocastration actionPeptide/protein ingredientsDigestive systemHuman tumorHuman fertility
A pharmaceutical composition using natural gonadotropin-releasing hormone (GnRH), and / or some of its mimetic peptides, indistinctly bound by its amino or carboxyl extremes to a carrier molecule; in one case by its carboxyl extreme and in the other case by the amino terminal extreme, thus eliciting a faster and more potent immunological response against the endogenous GnRH hormone. This finally leads to the ablation of the GnRH and consequently of the rest of the involved hormones in the stream GnRH / LH-FSH / Testosterone-(estrogens). An advantage of this formulation consists on facilitating the exposition to the immune system of a greater number of epitopes of the GnRH or its mimetics, minimizing thus the steric hindrance produced by the carriers. This invention has a direct application in the castration of pets and animals of economic interest, in the control of human fertility as well as in the treatment of hormone-sensitive tumors, such as that of the prostate, the breast, ovary, the endometry, testicles, hypophysis, salivary glands and other kinds of human tumors.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
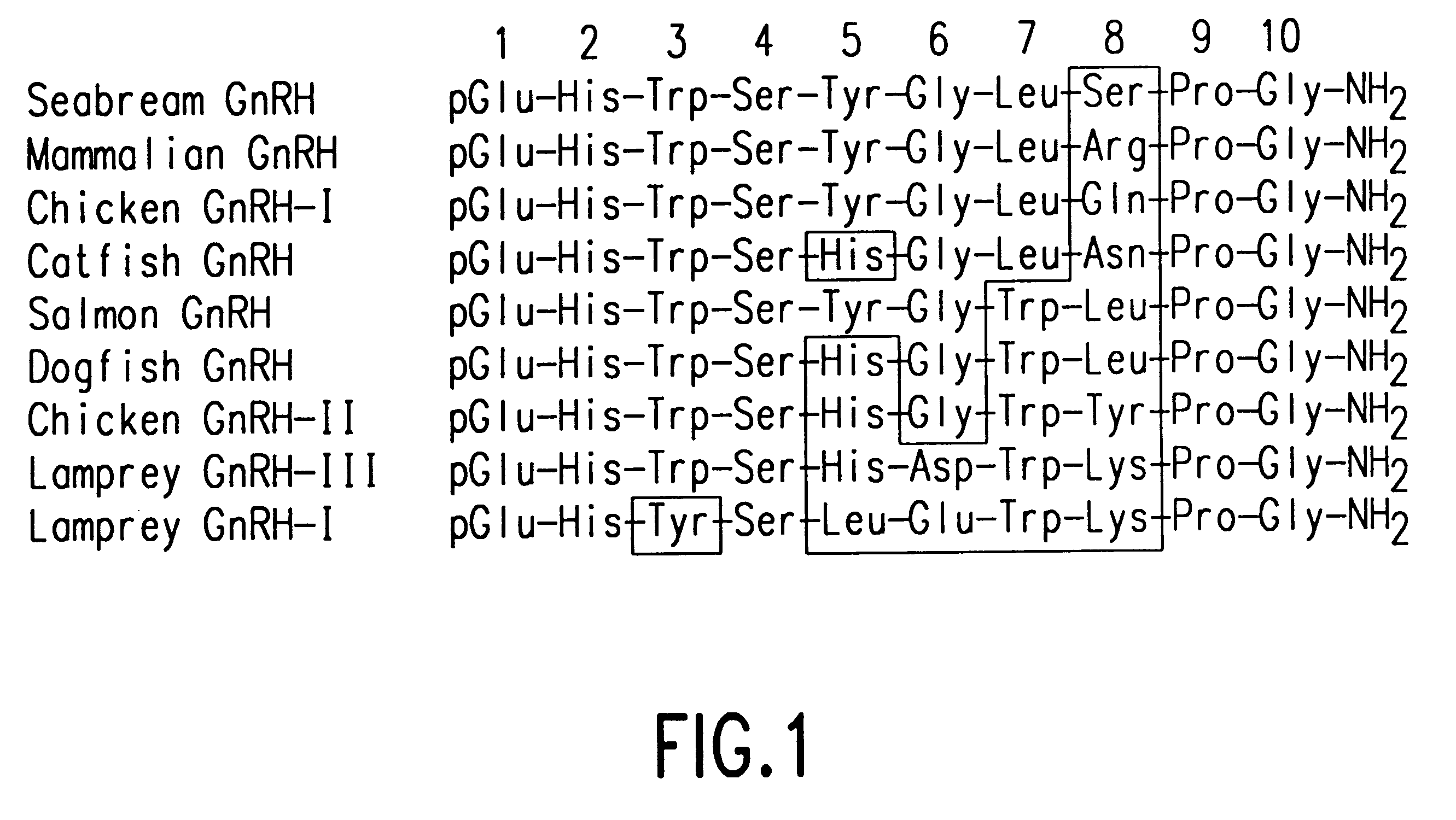
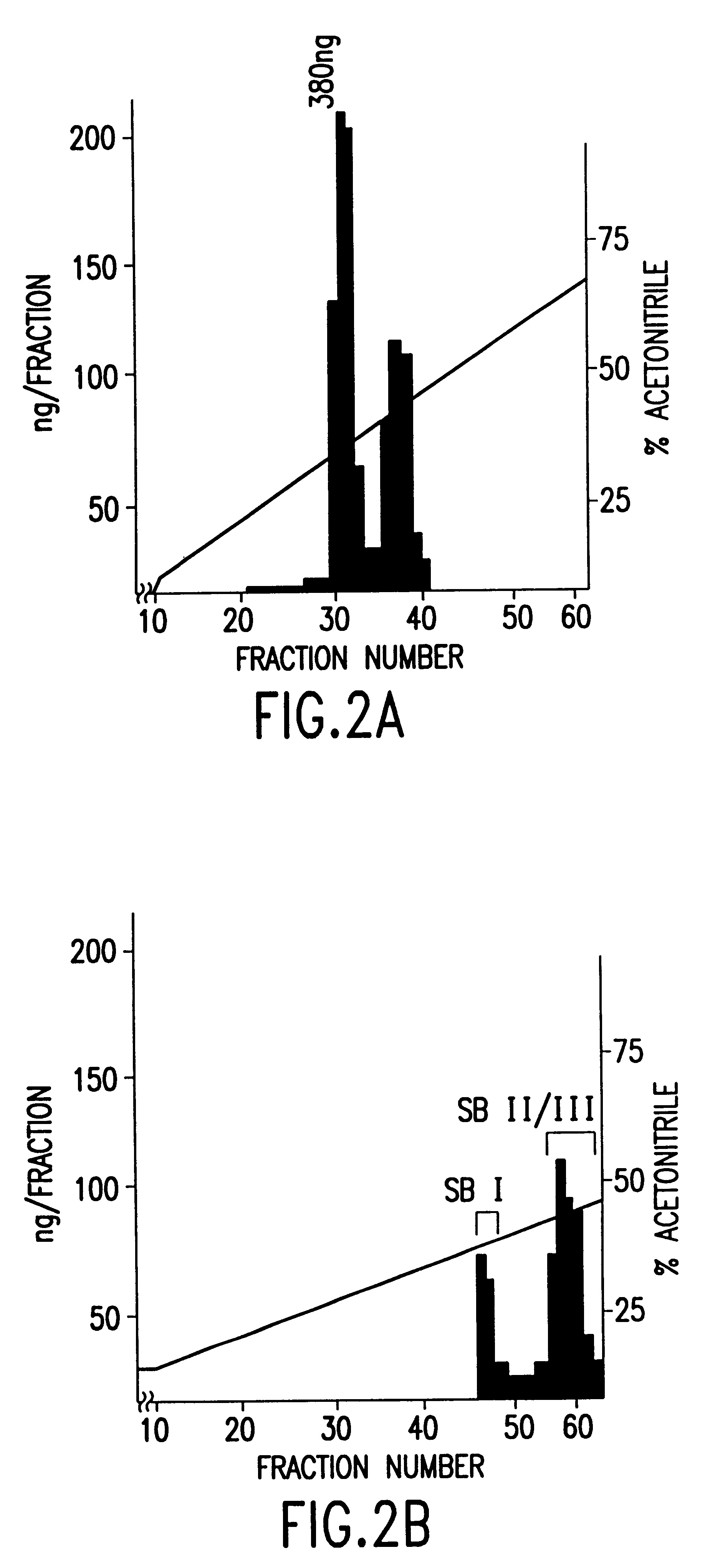
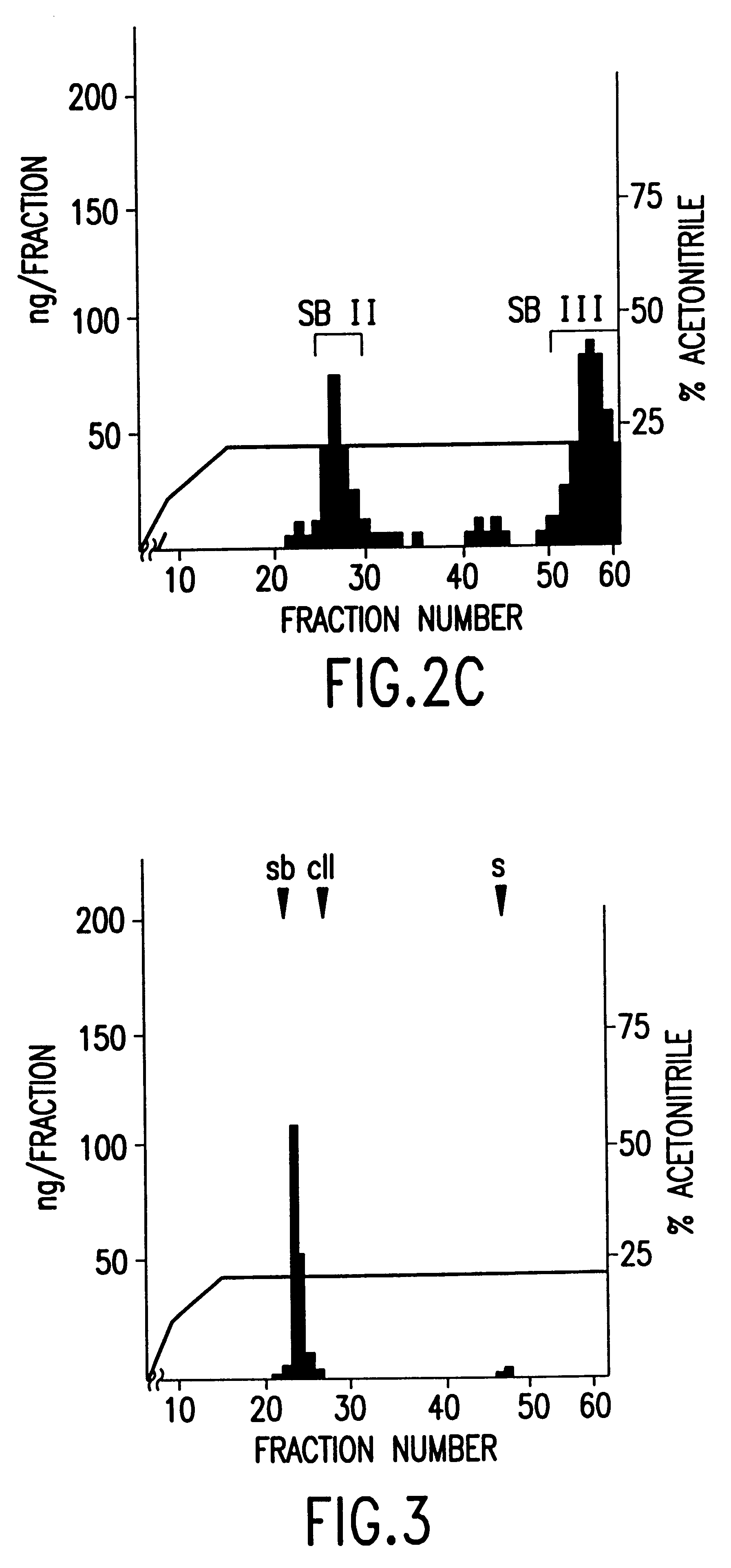
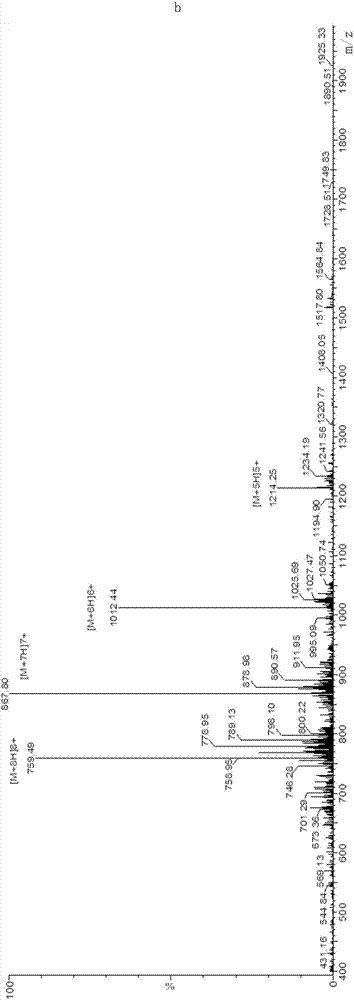
Polynucleotides encoding sbGnRH, a novel reproductive hormone
Novel peptide hormones which influence the release of gonadotropins by the pituitary gland in fish are disclosed. Methods in which such peptides may be administered to fish to control their reproduction are also described. Furthermore, novel isolated cDNA encoding the precursor of the novel gonadotropin-releasing hormone is disclosed. The use of such cDNA in controlling the gonadal development and spawning of fish is also described.
Owner:UNIV OF MARYLAND BIOTECH INST
GnRH antigen and application thereof in active immunization affecting castration effect and meat quality of oxen
ActiveCN106986923AImproving immunogenicityEnhance antigen immunogenicityVertebrate antigen ingredientsLuteinising hormone-releasing hormoneActive immunizationMale rats
The invention discloses a GnRH antigen and application thereof in active immunization affecting the castration effect and meat quality of oxen. The invention provides a GnRH derivative which is obtained by inserting oligopeptides, which can form alpha helixes, among multiple serially-connected single GnRH antigens, and each single GnRH antigen is a polypeptide obtained by replacing the sixth-site glycine in the amino acid sequence of gonadotropin releasing hormone GnRH with D type lysine. When a GnRH two-string alpha-helix vaccine screened by experiments is used to actively immunize male rats and the oxen, the vaccine can allow the biological activity of testosterone to be partially or completely lost, an immunocastration effect is achieved, a good immunization enhancing effect is achieved, and the carcass quality of immunized animals can be increased to a certain degree.
Owner:XINJIANG ACADEMY OF AGRI & RECLAMATION SCI
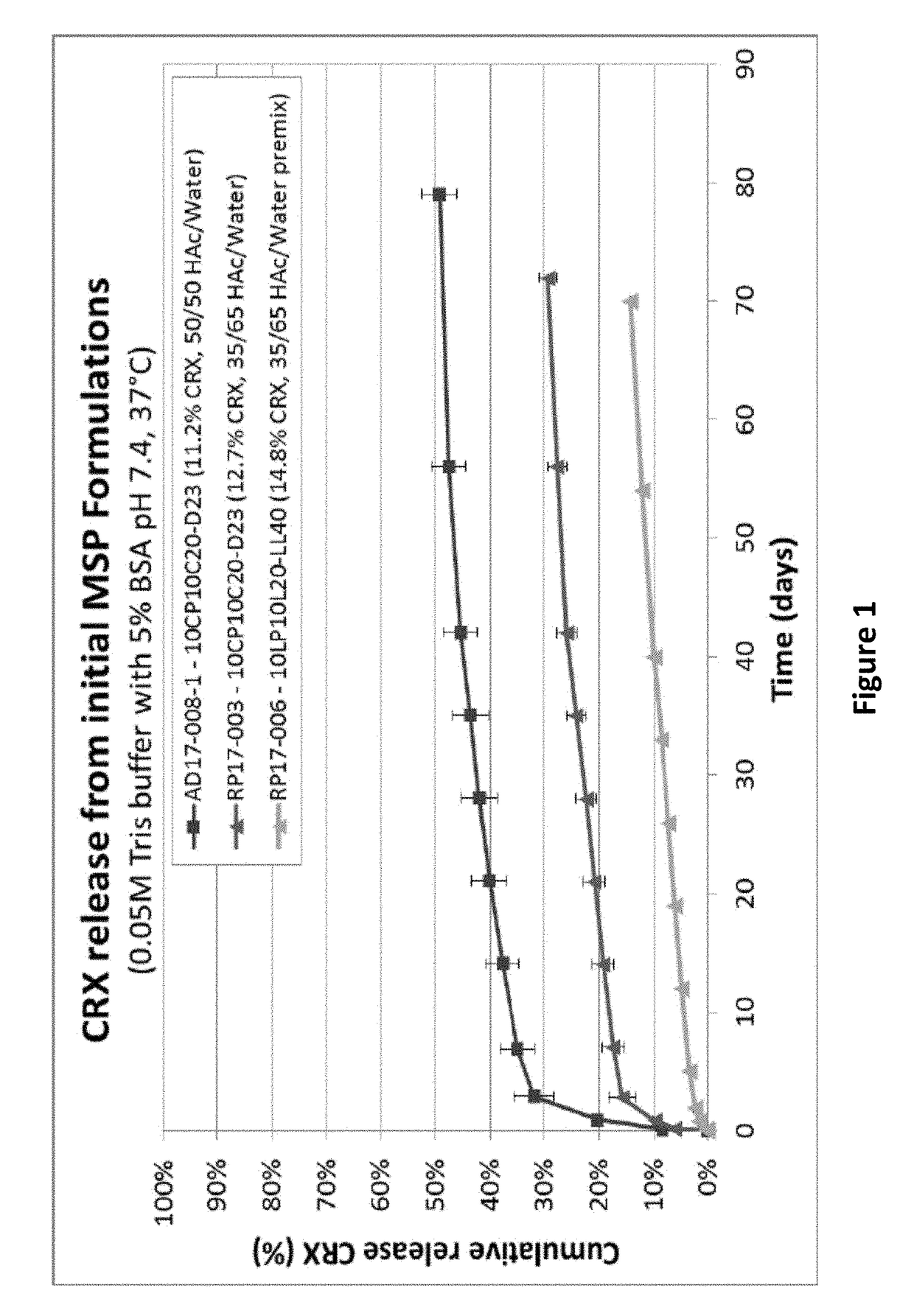
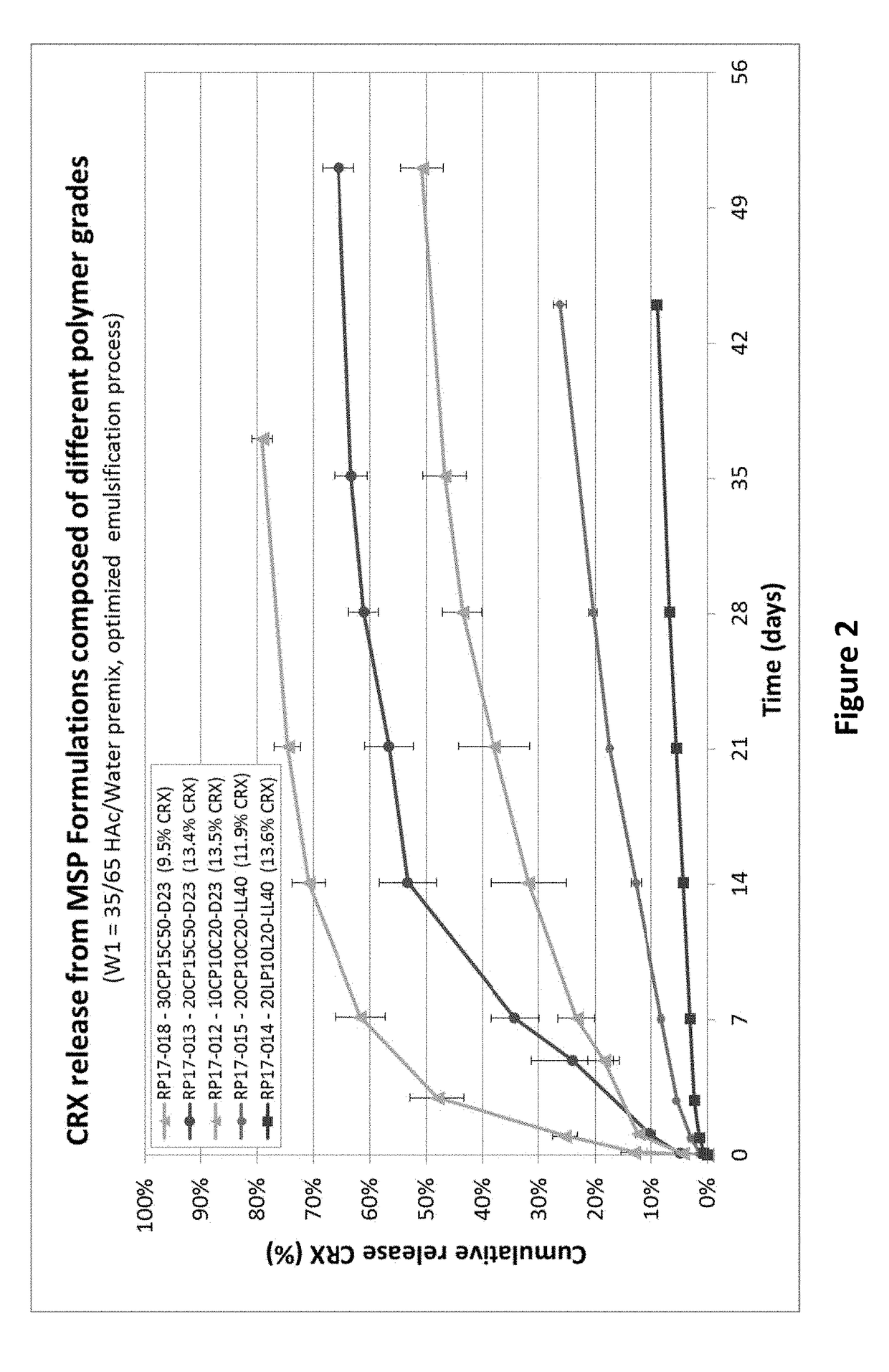
COMPOSITIONS AND METHODS FOR LONG TERM RELEASE OF GONADOTROPIN-RELEASING HORMONE (GnRH) ANTAGONISTS
PendingUS20180214507A1Peptide/protein ingredientsOintment deliveryGonadotropin-releasing hormone antagonistControl release
The invention provides compositions and methods for long term release of Gonadotropin-releasing hormone (GnRH) antagonists, and uses thereof. Specifically, the invention provides polymer compositions and methods for controlled release of GnRH antagonists.
Owner:THE FEMALE HEALTH CO D B A VERU
Compositions suitable for controlled release of the hormone GnRH and its analogs
InactiveUS20060025328A1Easily-injectable solutionReduce molecular weightPeptide/protein ingredientsPharmaceutical delivery mechanismControl releasePhysiology
A liquid composition for the controlled release of gonodotropin releasing hormone (GnRH) or its analogs or agonists is provided that includes: (i) a non-polymeric, non-water soluble liquid carrier material (HVLCM) of viscosity of at least 5,000 cP at 37° C. that does not crystallize neat under ambient or physiological conditions; and (ii) GnRH or analogs agonists thereof. The composition can be used to treat reproductive conditions and / or induce ovulation in animals, such as livestock, fish, and shellfish.
Owner:BURNS PATRICK J
Artificial decapeptide for inducing vitellogenesis in fish
ActiveUS20110226188A1Promote sexual maturation and vitellogenesisAnimal reproductionPeptide/protein ingredientsFisheryVitellogenesis
An artificial decapeptide for inducing vitellogenesis in fish, being an analogue of gonadotropin-releasing hormone of chicken is set forth in SEQ ID NO: 2. The artificial decapeptide can be further developed and manufactured into a preparation, which is capable of inducing vitellogenesis in fish. With the implantation of the preparation into body cavity or body wall of bony fish, the induction of vitellogenesis and ovum maturation in bony fish can be successfully achieved.
Owner:NAT PINGTUNG UNIV OF SCI & TECH
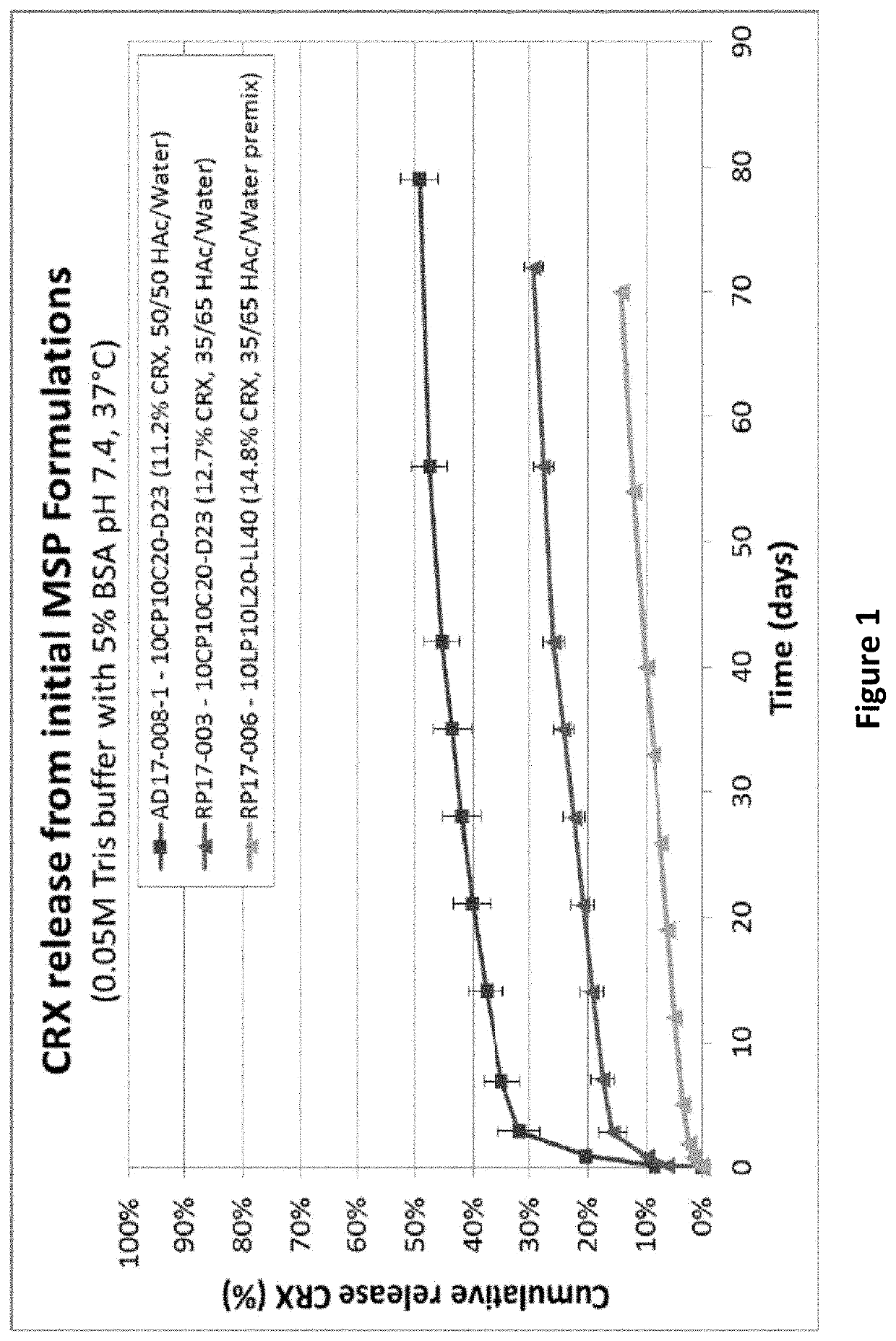
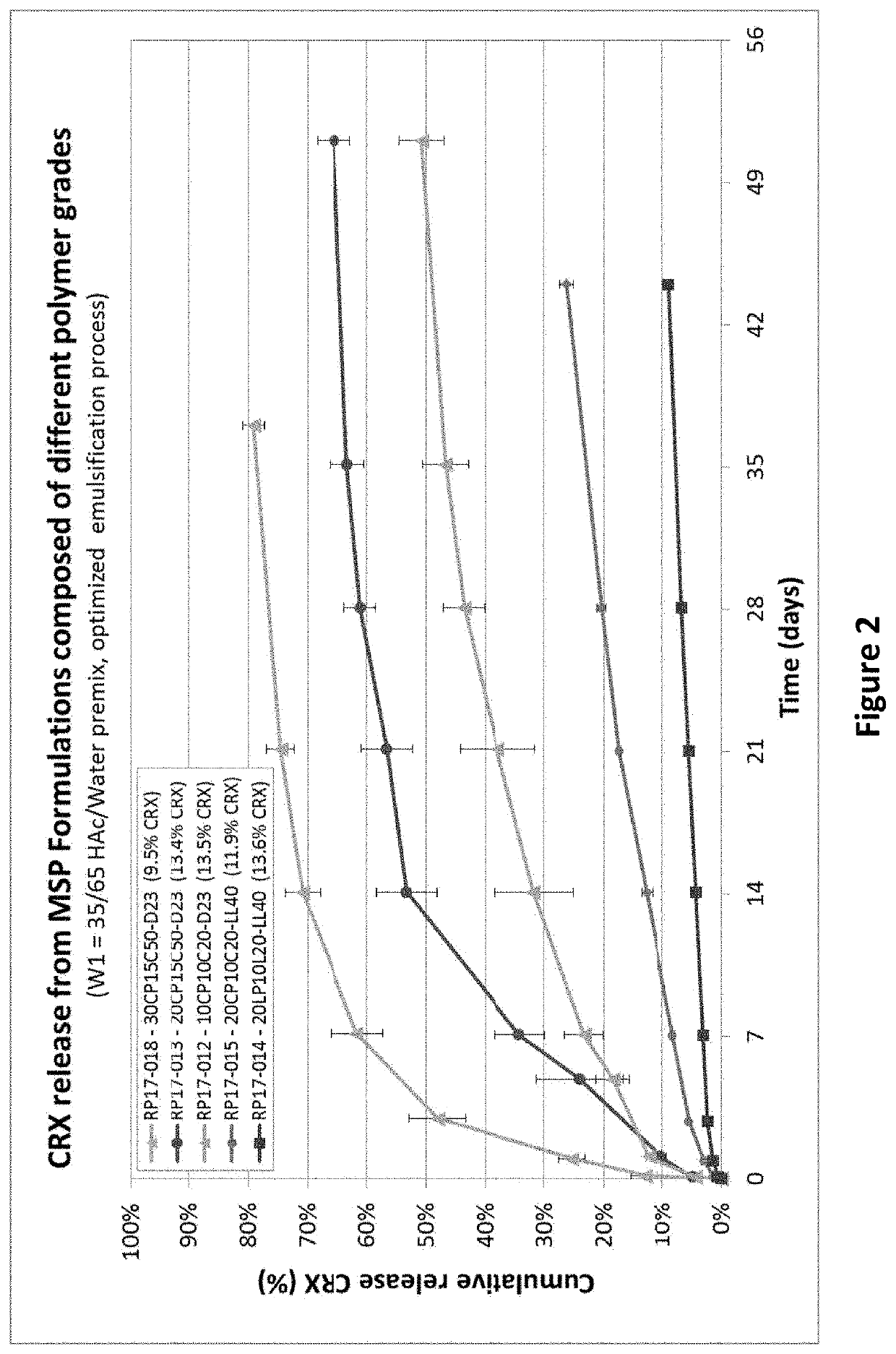
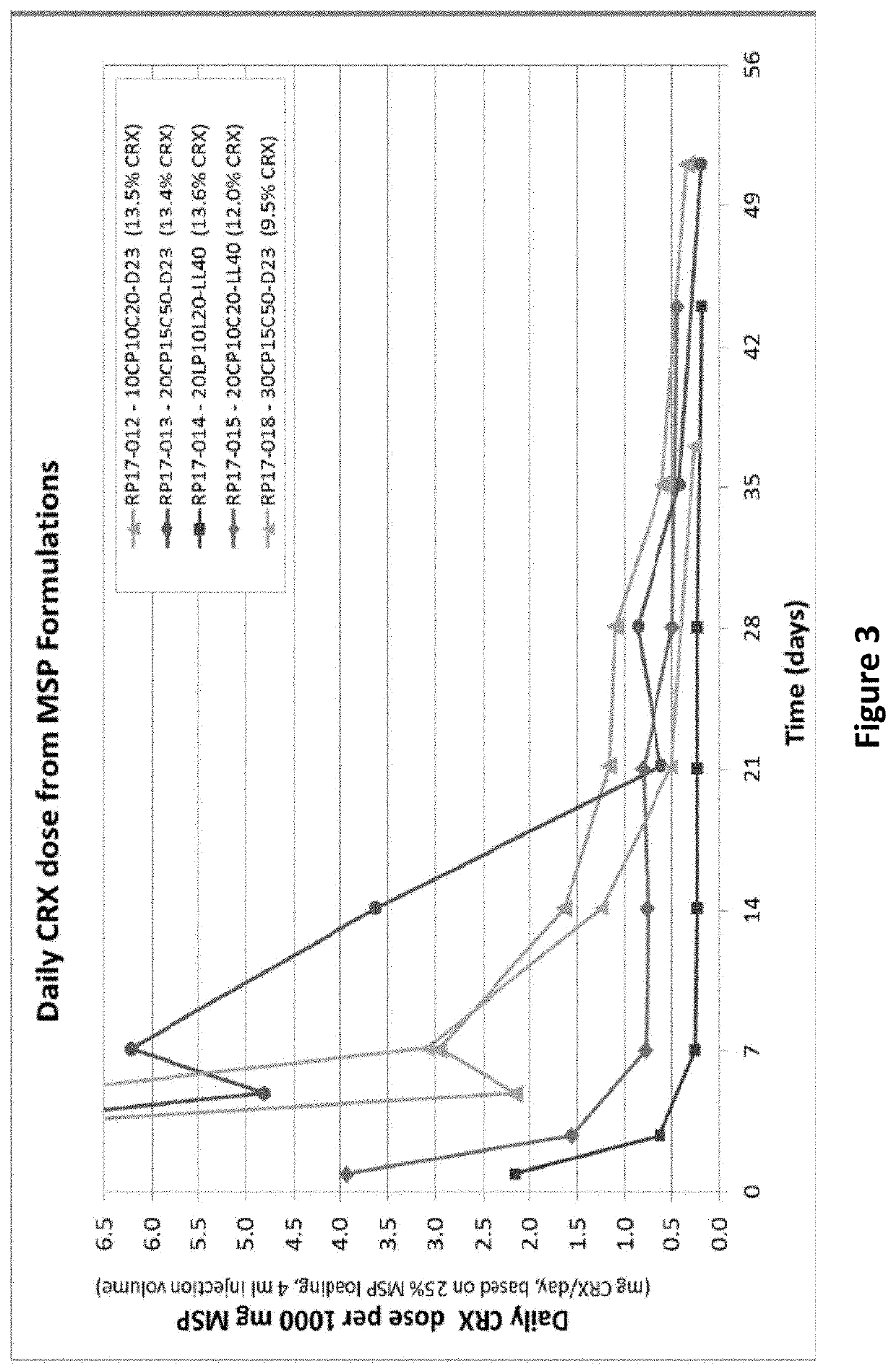
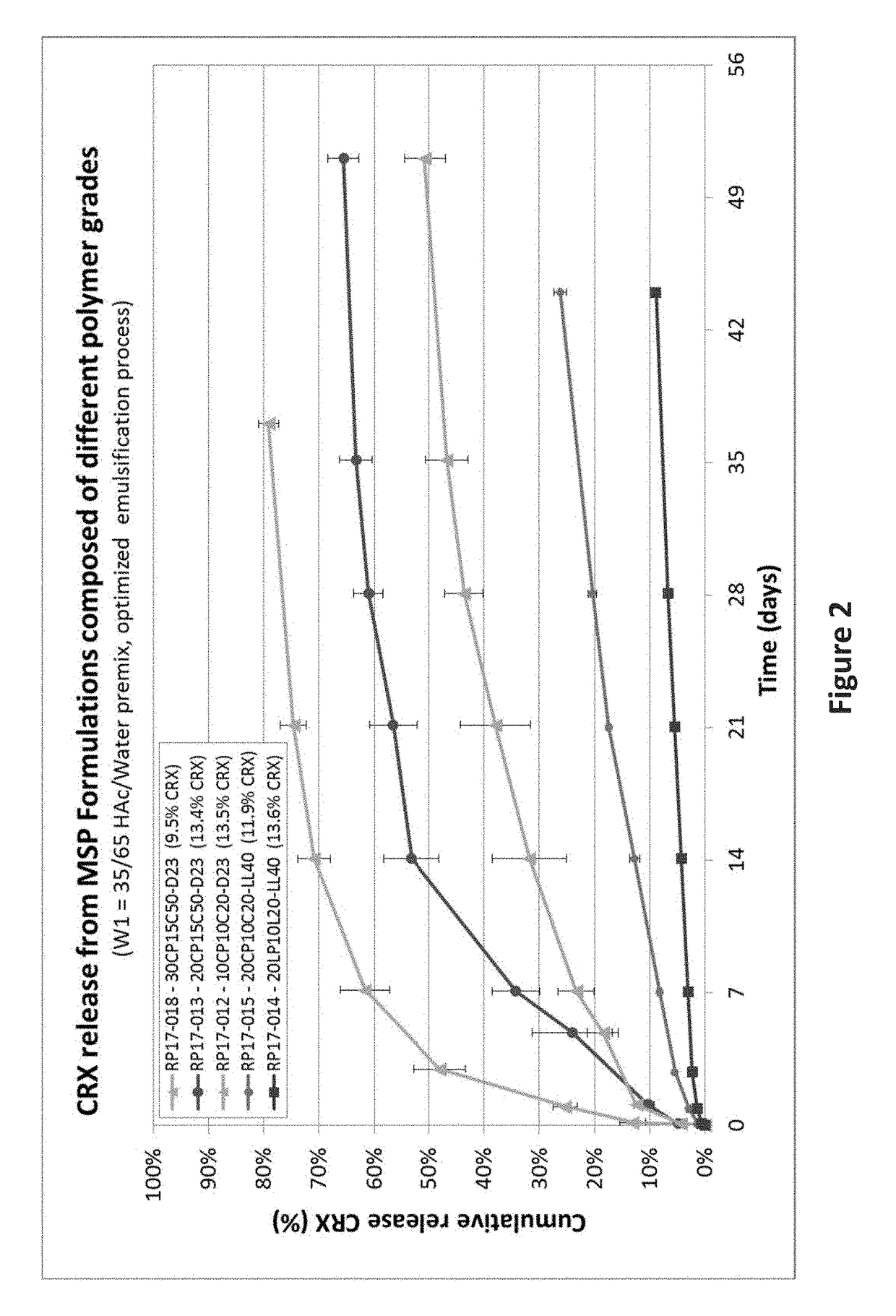
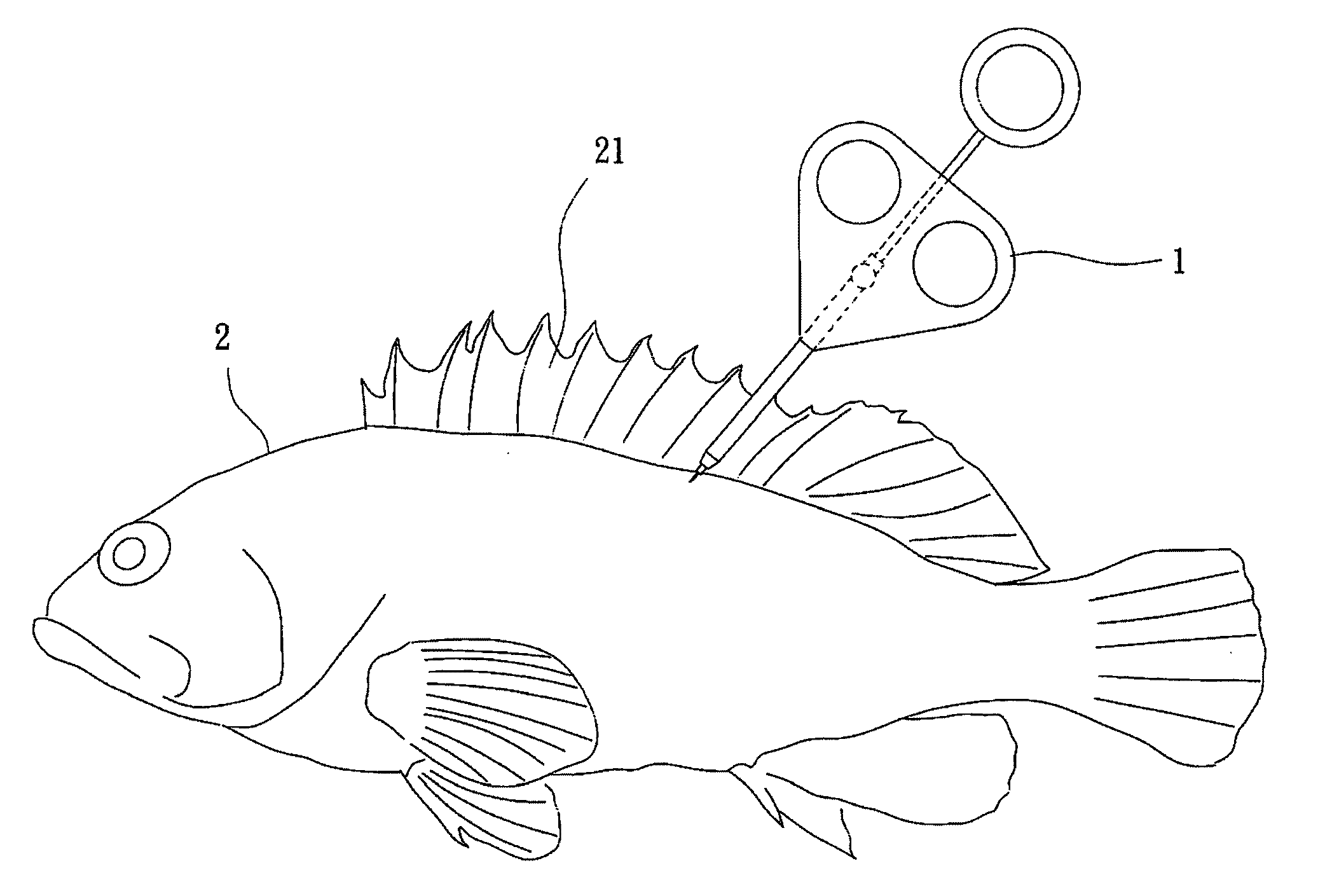
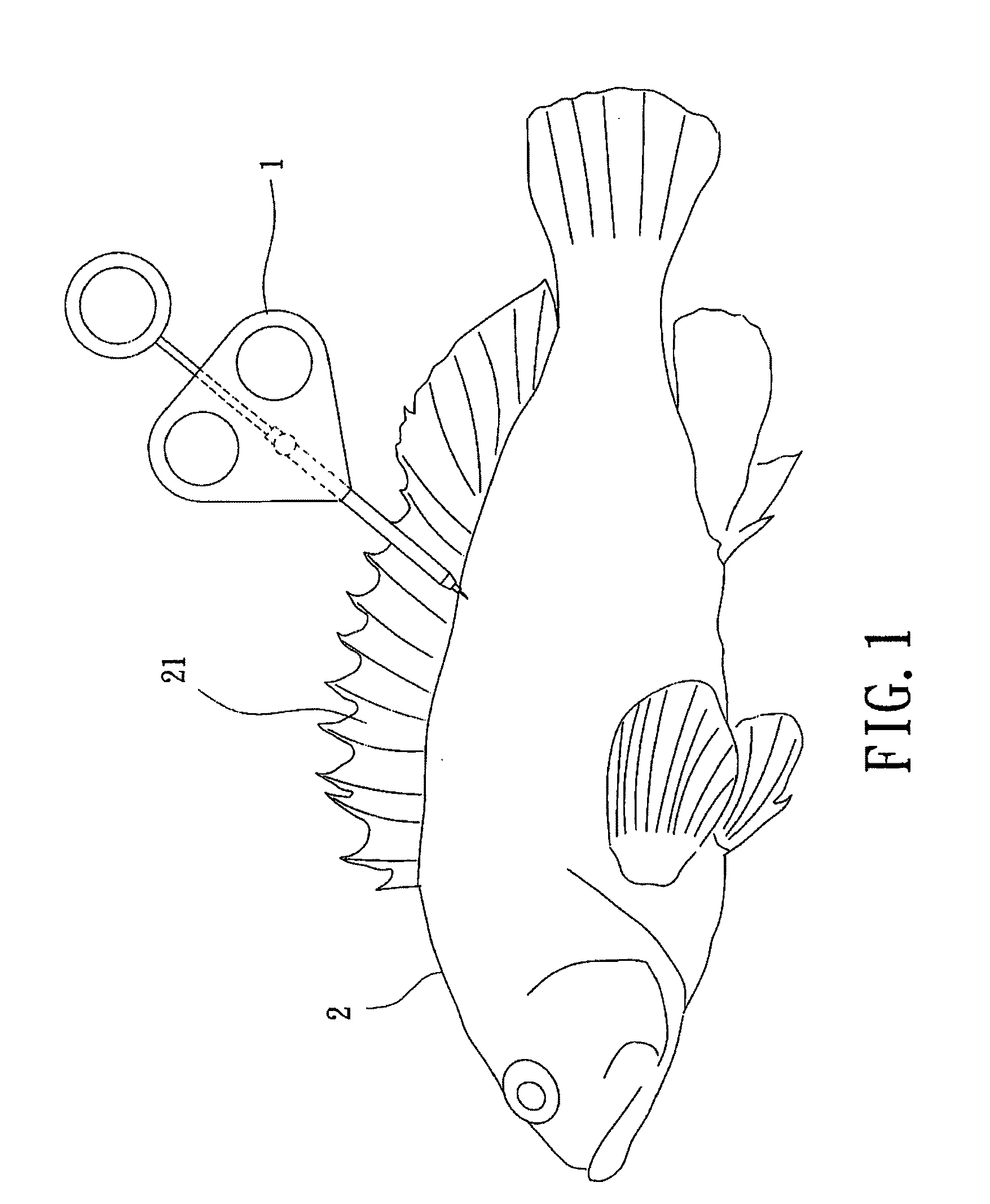
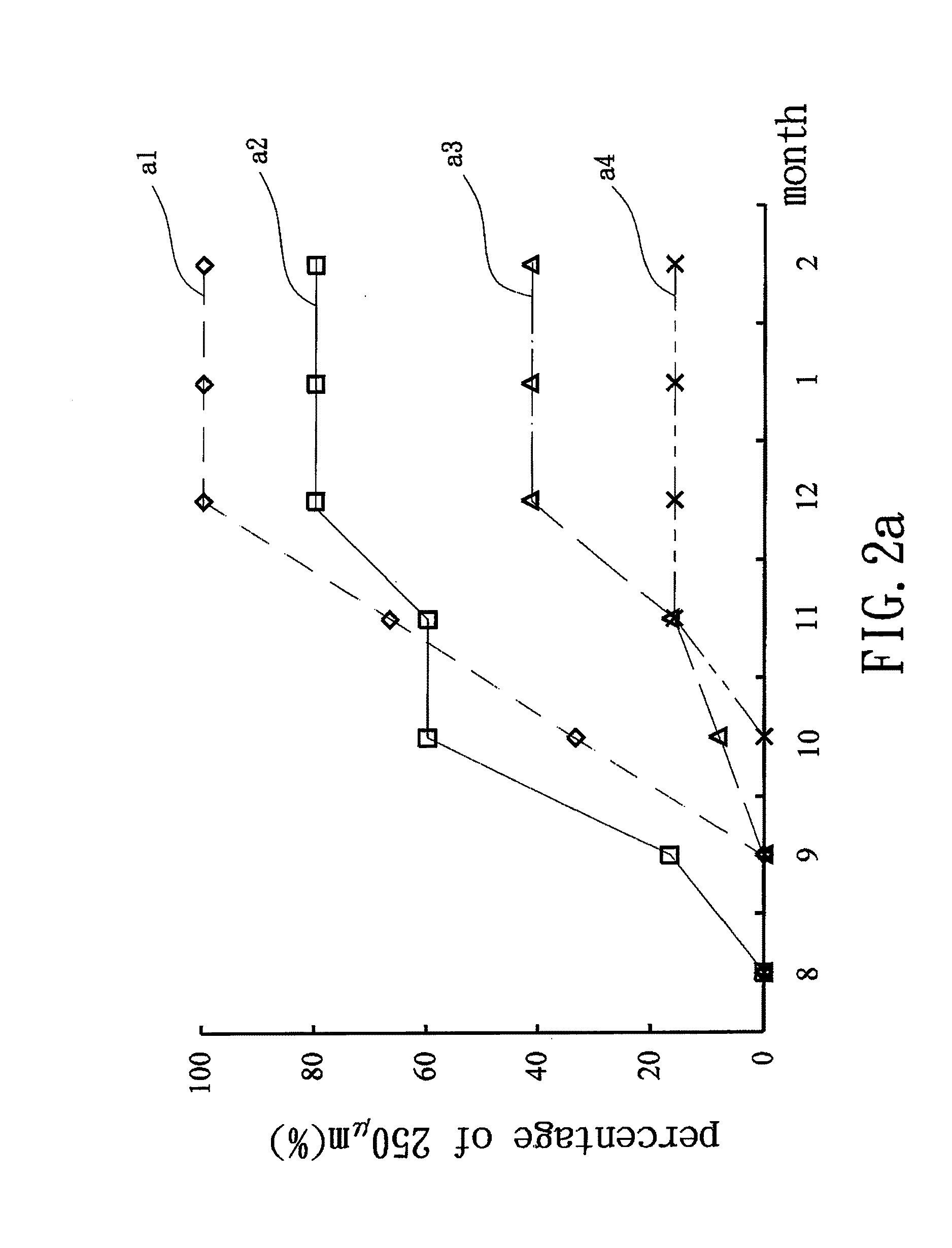
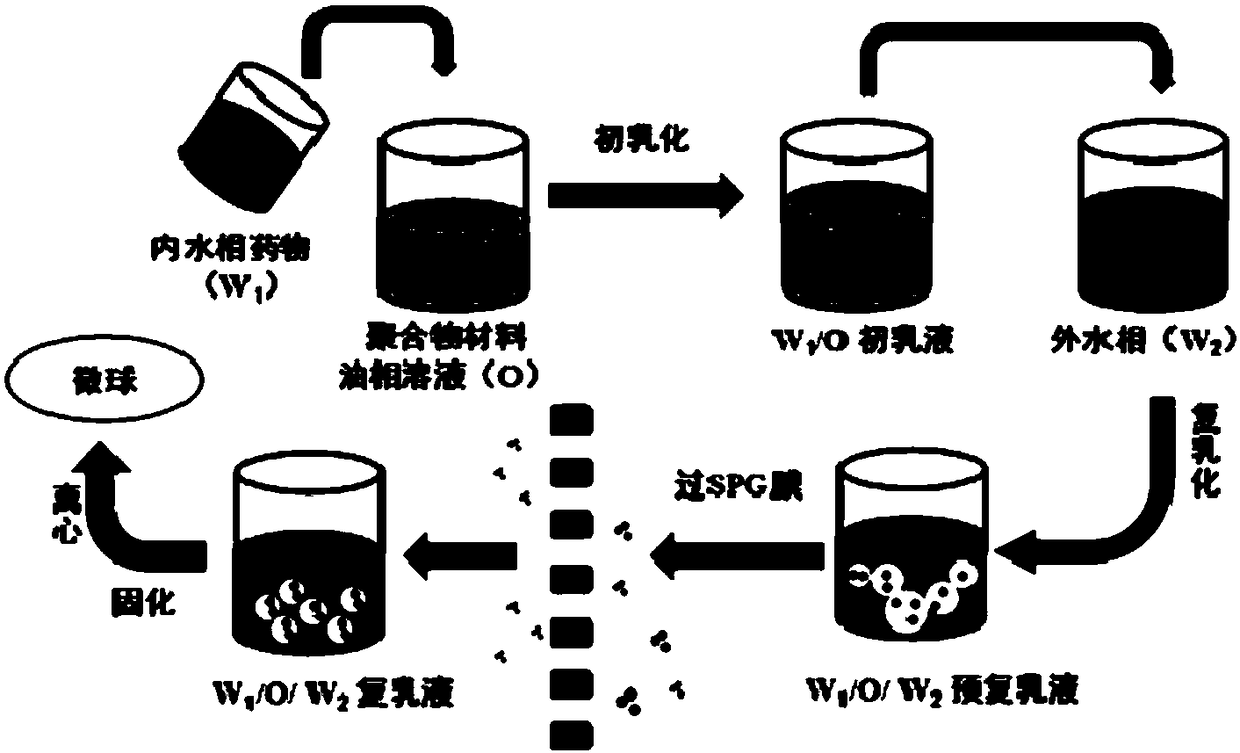
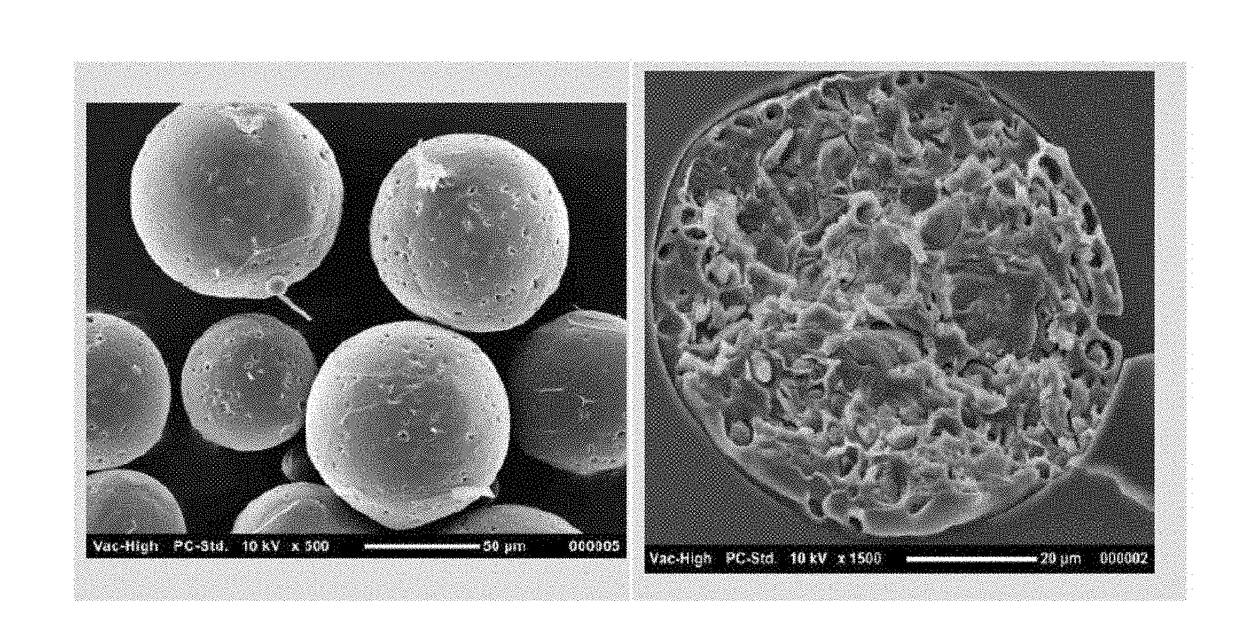
Drug sustained release microsphere, preparation method and application thereof
ActiveCN108969751AGood repeatabilityReduce diffusePeptide/protein ingredientsAntineoplastic agentsMicrosphereOil phase
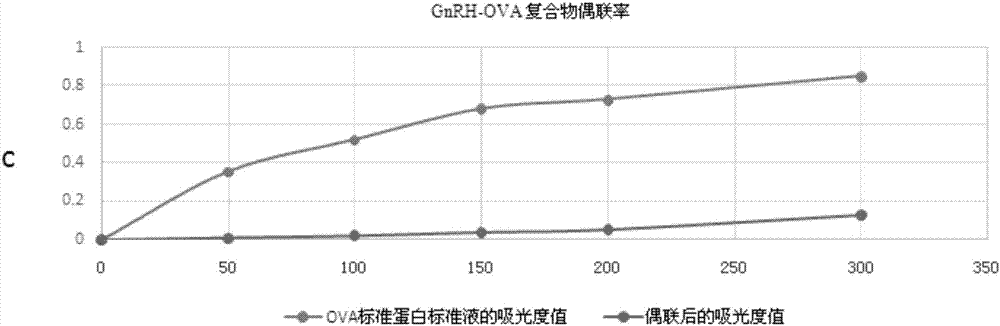
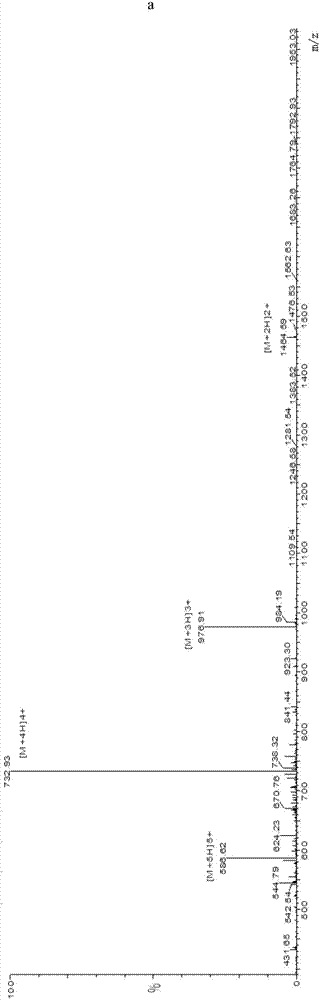
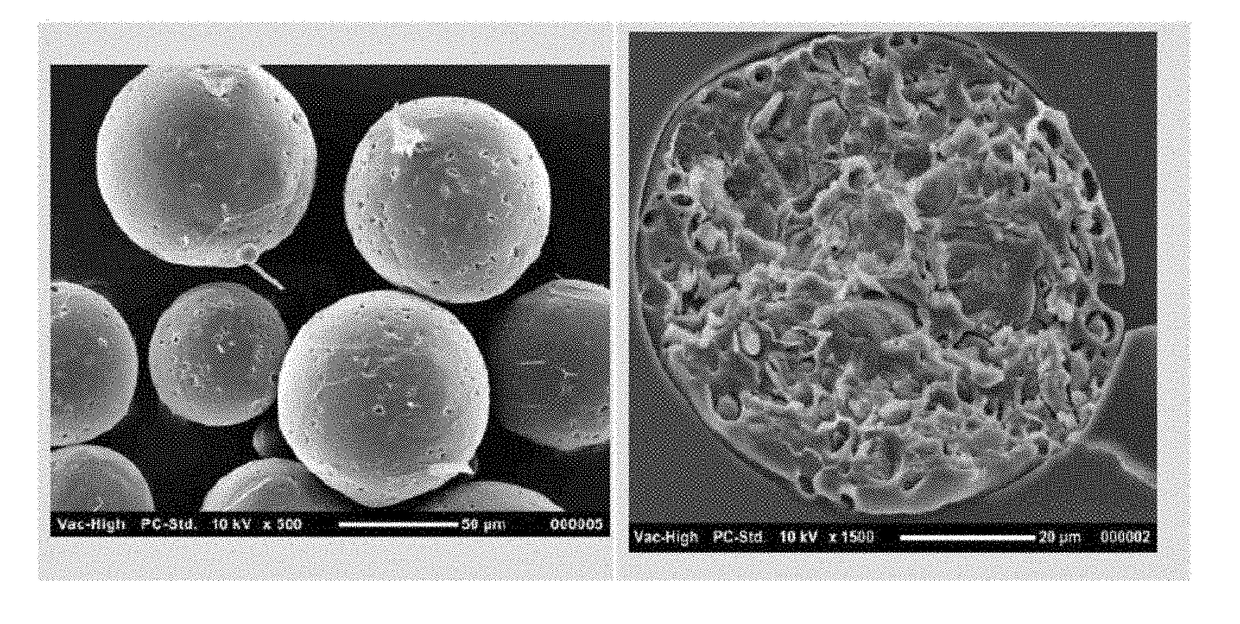
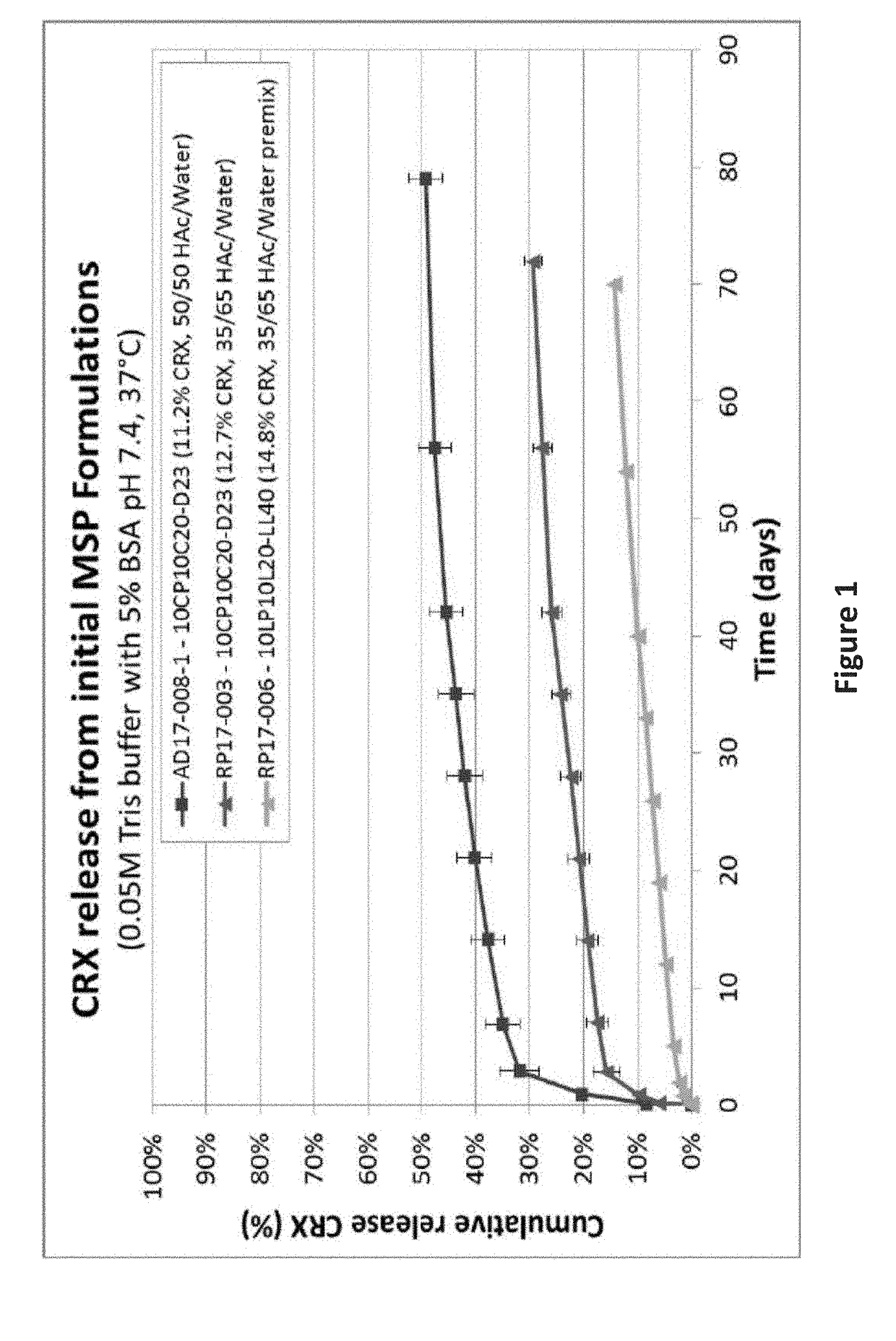
The invention provides a drug sustained release microsphere, a preparation method and an application thereof. The preparation method comprises the following steps: filtering an 'internal water phase / oil phase / external water phase' pre-emulsion solution with a micro-pore film, and then removing a solvent during a curing process, washing and drying, thereby acquiring the drug sustained release microsphere, wherein a gonadotrophin releasing hormone aqueous solution is served as the internal water phase. The preparation method provided by the invention is simple. The drug sustained release microsphere prepared according to the invention has uniform and controllable size, different batches of products are high in repeatability and the drug sustained release microsphere is easy for industrial production.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
METHOD OF TREATING PROSTATE CANCER WITH GnRH ANTAGONIST
InactiveUS20090203623A1Safely and rapidly suppress testosterone levelReduce riskPeptide/protein ingredientsPharmaceutical delivery mechanismUpper urinary tract infectionSide effect
The invention provides methods and dosing regimens for safely and effectively treating androgen-dependent prostate cancer with a gonadotrophin releasing hormone (GnRH) antagonist without causing a testosterone spike and / or other side effect of GnRH agonist therapy such as a urinary tract infection, or an arthralgia-related or cardiovascular side effect.
Owner:FERRING BV
COMPOSITIONS AND METHODS FOR LONG TERM RELEASE OF GONADOTROPIN-RELEASING HORMONE (GnRH) ANTAGONISTS
PendingUS20180250354A1Peptide/protein ingredientsOintment deliveryGonadotropin-releasing hormone antagonistControl release
The invention provides compositions and methods for long term release of Gonadotropin-releasing hormone (GnRH) antagonists and uses thereof. Specifically, the invention provides polymer compositions and methods for controlled release of GnRH antagonists.
Owner:VERU INC
Veterinary composition and methods for non-surgical neutering and castration
ActiveUS9770011B2Great tasteLower Level RequirementsOrganic active ingredientsPowder deliveryPhysiologyGonadal Steroid Hormones
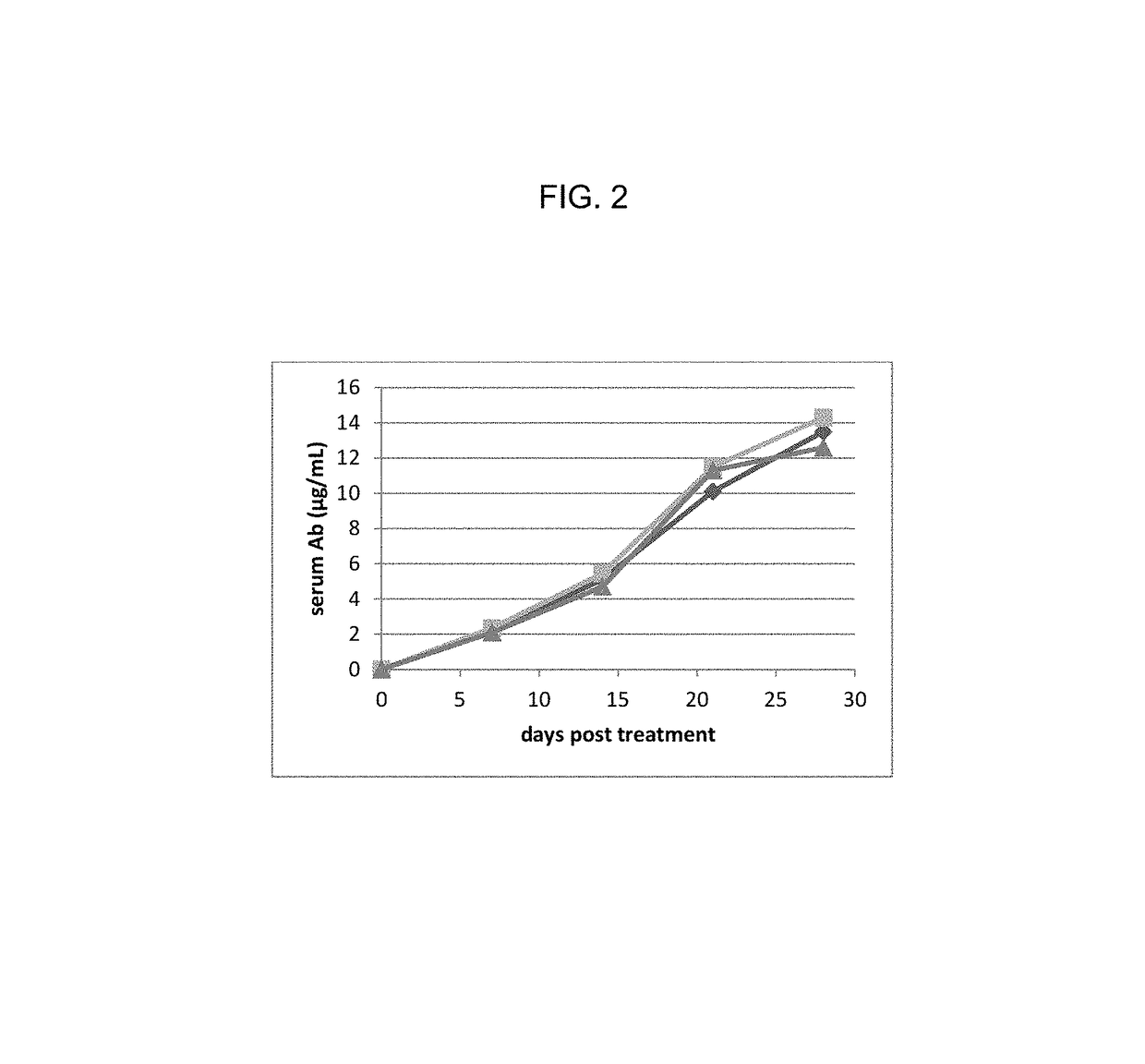
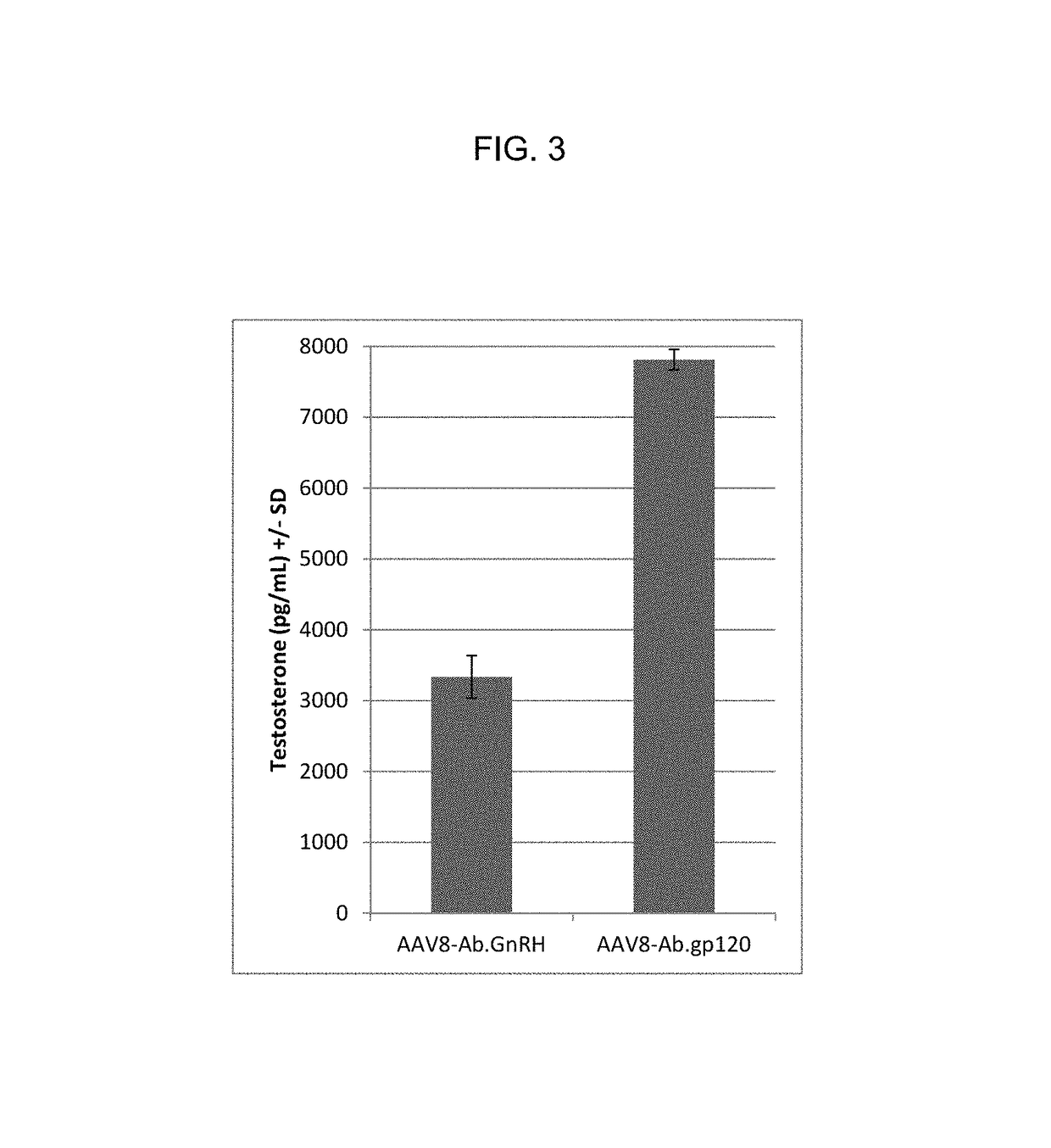
A method for non-surgical neutering or castration of a non-human mammal for AAV-mediated delivery of an anti-GnRH polypeptide to a non-human animal is described. More particularly, the animal is administered an adeno-associated virus (AAV) vector having an AAV capsid having packaged therein nucleic acid sequences comprising an AAV 5′ inverted terminal repeat (ITR), a sequence encoding a polypeptide which specifically binds gonadotropin releasing hormone (GnRH) under control of regulatory sequences which direct expression of the polypeptide, and an AAV 3′ ITR. A composition comprising the AAV-anti-GnRH may also be used for inhibiting tumor growth in a mammal with a cancer responsive to gonadal steroid hormones.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Thienopyridine compounds, their production and use
InactiveUS6297255B1Easy to useHigh activityBiocideOrganic chemistryEccentric hypertrophyPrecocious puberty
The compound of the present invention possesses excellent gonadotropin-releasing hormone antagonizing activity, and is useful for preventing or treating sex hormone-dependent diseases, e.g., sex hormone-dependent cancers (e.g., prostatic cancer, uterine cancer, breast cancer, pituitary tumor), prostatic hypertrophy, hysteromyoma, endometriosis, precocious puberty, amenorrhea syndrome, multilocular ovary syndrome, pimples etc. or as a pregnancy regulator (e.g., contraceptive), infertility remedy or menstruation regulator.
Owner:TAKEDA PHARMA CO LTD
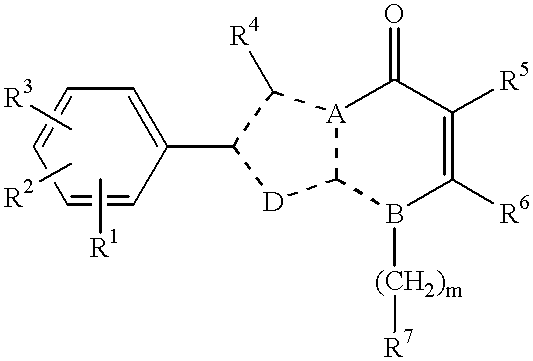
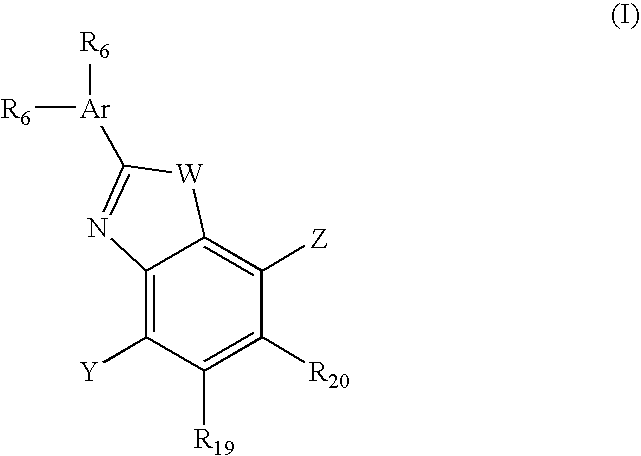
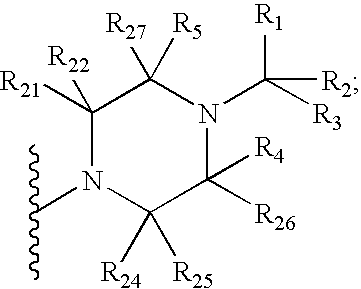
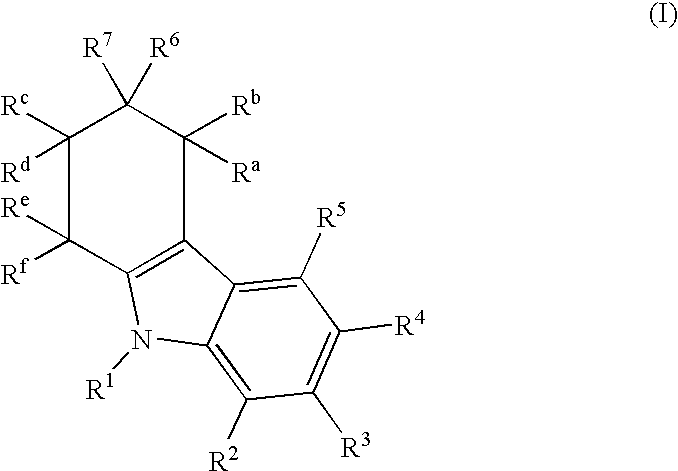
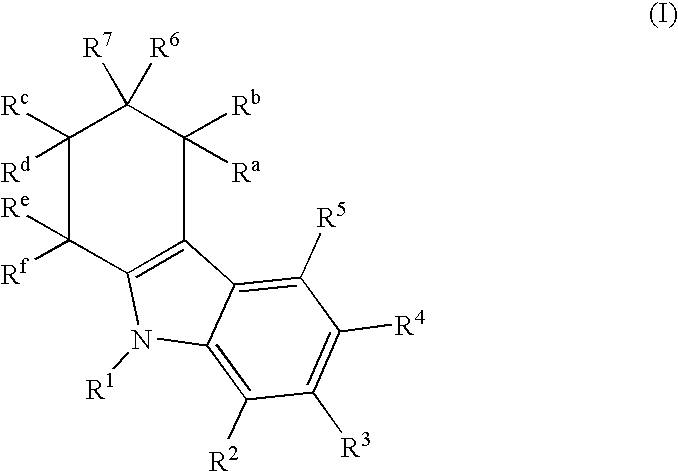
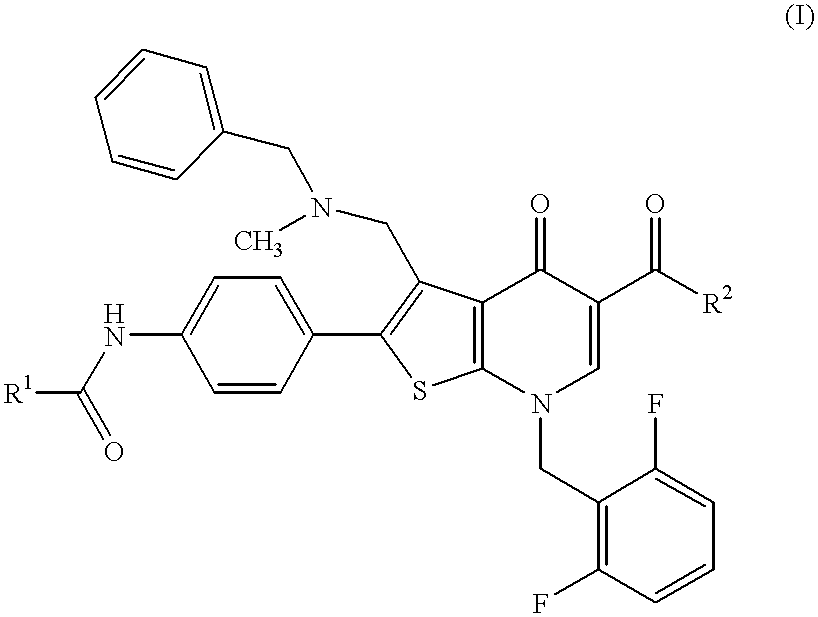
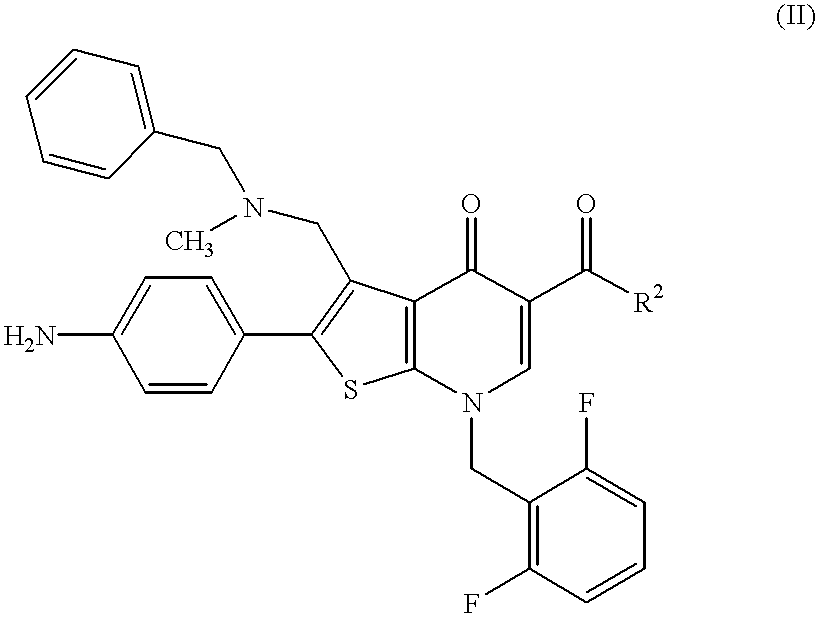
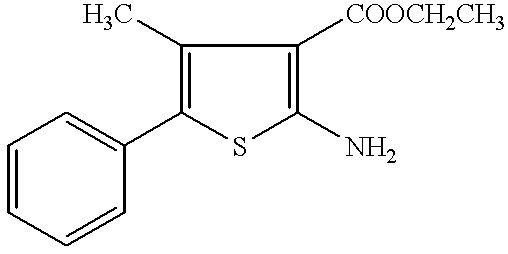
Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor
InactiveUS7534796B2BiocideOrganic chemistryGonadotropin-releasing hormone receptorHormones regulation
The present invention relates to Gonadotropin Releasing Hormone (GnRH, also known as Luteinizing Hormone Releasing Hormone) receptor antagonists.
Owner:WYETH LLC
Gonadotropin Releasing Hormone Receptor Antagonist, Preparation Method Thereof And Pharmaceutical Composition Comprising The Same
InactiveUS20130137661A1BiocideNervous disorderGonadotropin-releasing hormone receptorNK1 receptor antagonist
Owner:TIUMBIO CO LTD
Nitrogen-containing heterocyclic compounds, their production and use
InactiveUS6413972B1Excellent GnRH-antagonizing activityEasy to useOrganic active ingredientsBiocideDiseaseNitrogenous heterocyclic compound
Owner:TAKEDA PHARMA CO LTD
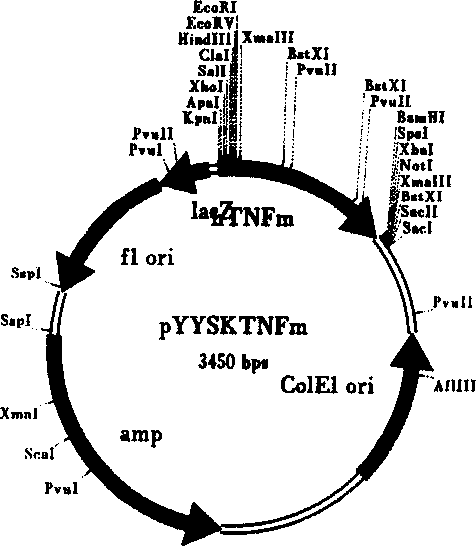
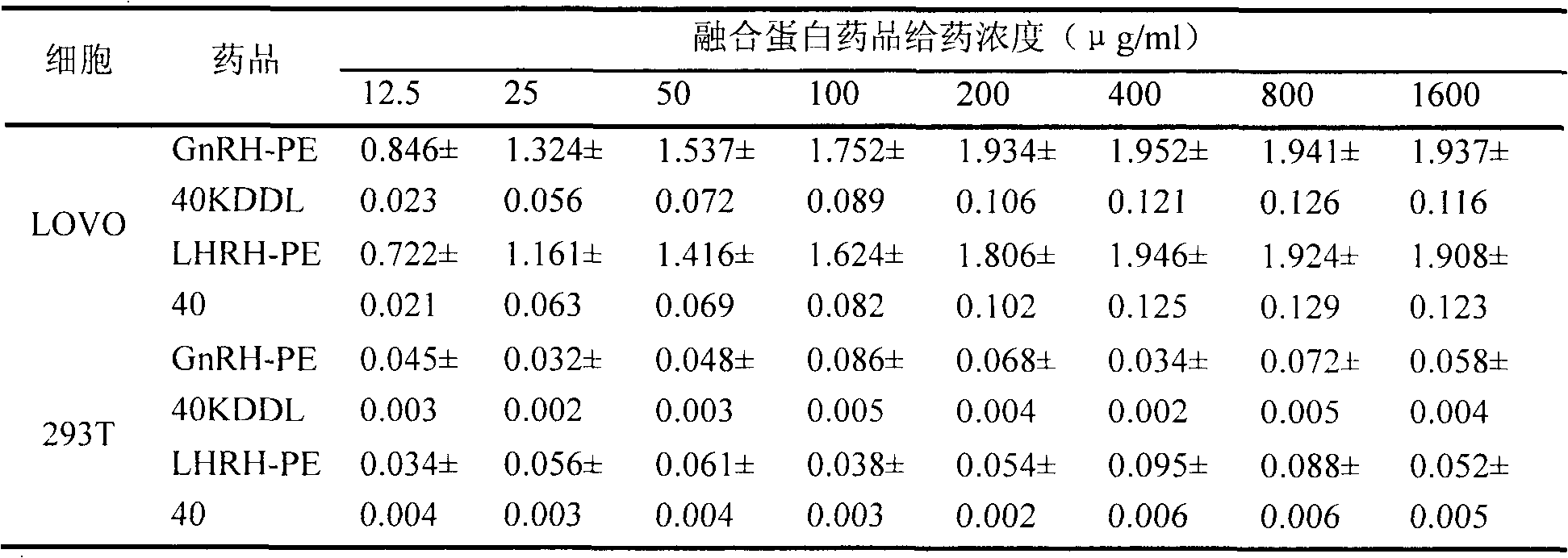
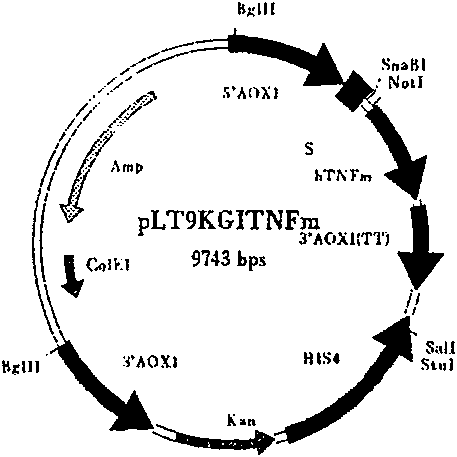
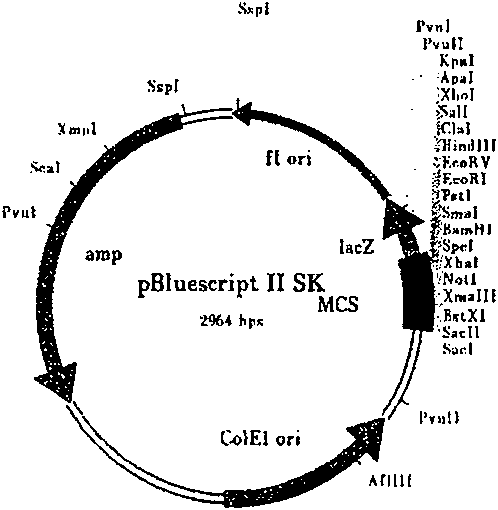
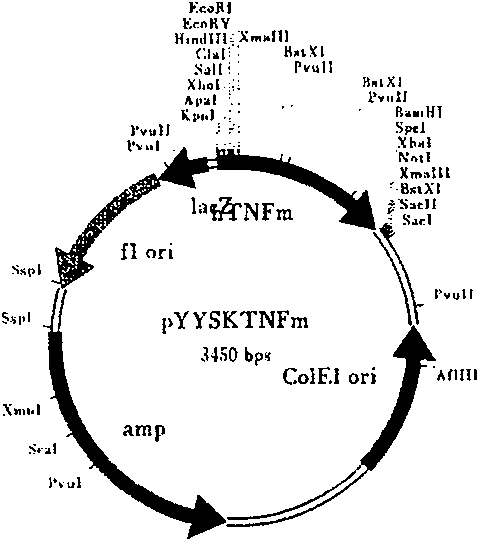
Recombinant target fusion protein GnRH-TNFam and its antitumor use
The invention provides a novel recombination targeting fusion protein GnRH-TNF alpham which is integrated from gonadotrophin releasing hormone (GnRH-A) and recombinant mutant human tumor necrosis factor alpha (TNF alpha), the targeting fusion protein has destroying action to the tumor cells endured by the prototype TNF alpha. The invention also provides the DNA sequence of the recombinant target fusion protein GnRH-TNFam and its expression recombinants.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
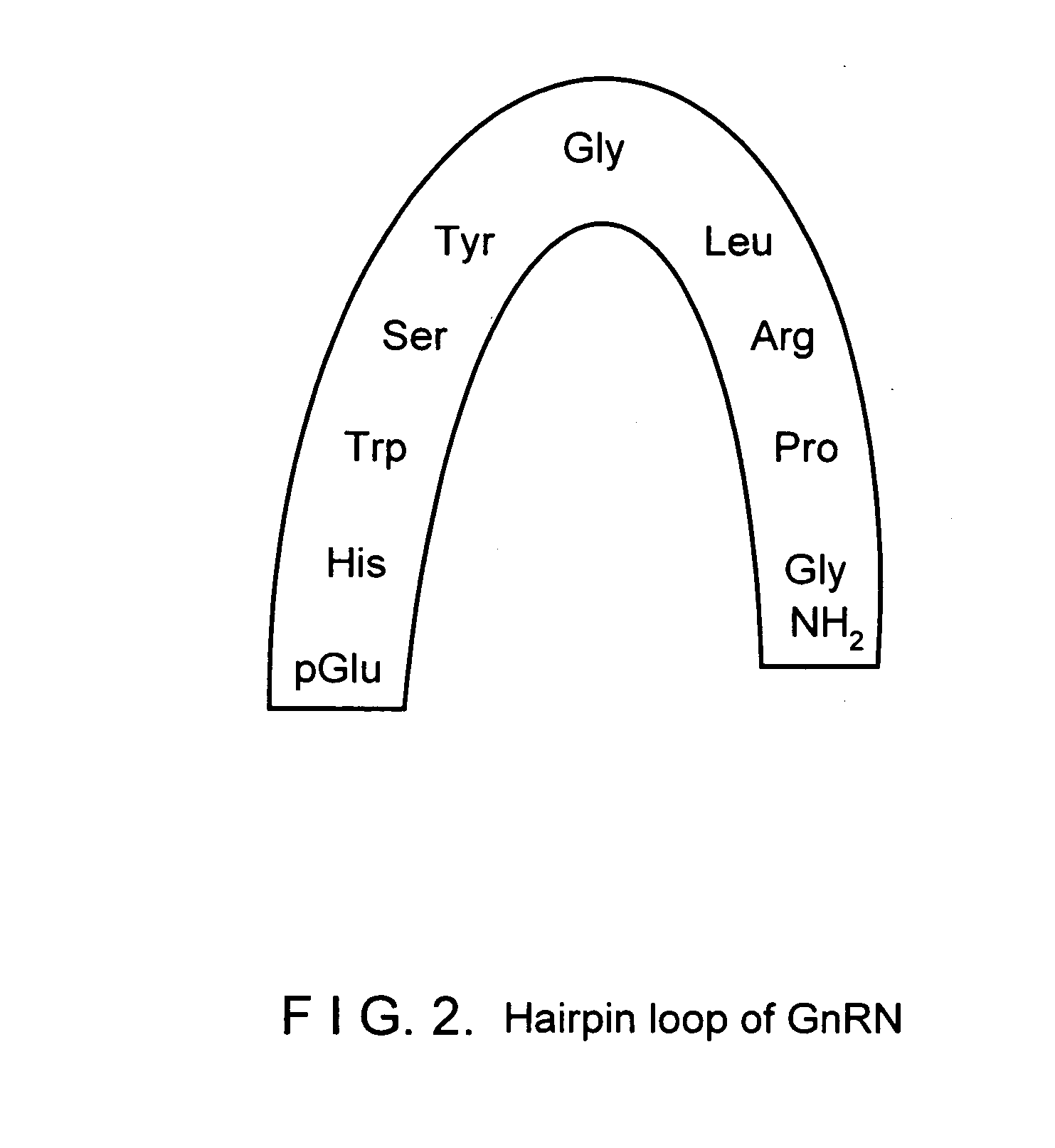
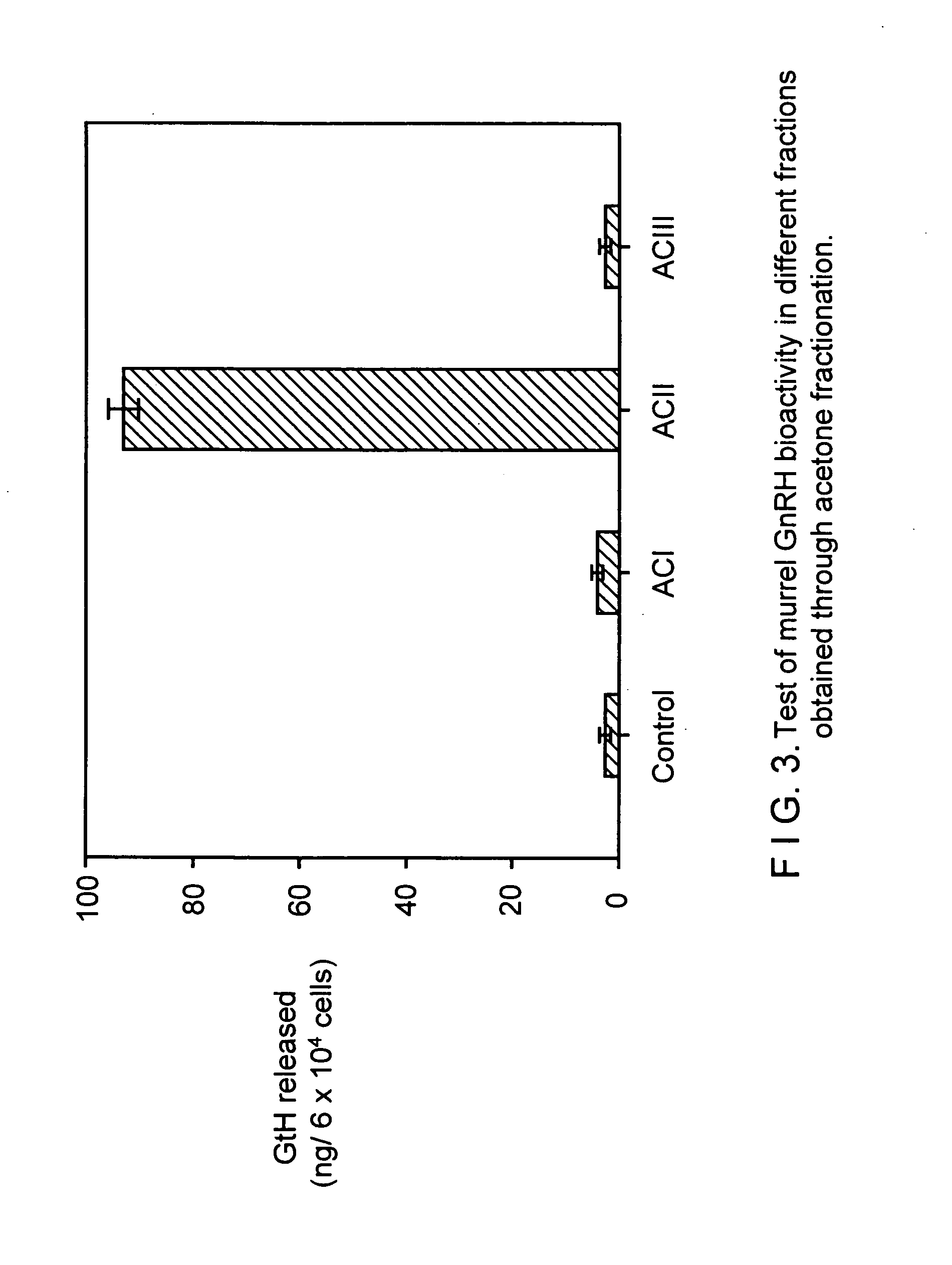
Novel two gonadotropin releasing hormones and a method to isolate the same
InactiveUS20050233951A1Facilitated releasePeptide/protein ingredientsComponent separationBiologyGonadotropin-releasing hormone
The present invention relates to two novel Gonadotropin releasing hormones muGnRH I and muGnRH II of amino acid SEQ ID 1 as QHWSAWRLPG, and SEQ ID 2 QHWSWGILPG respectively, useful for induced breeding in fish both in combination and alone, by activating production of Gonadotropin, and a method of isolating the same from Indian Murrel brain, and further, a method of inducing breeding in fishes using the said novel gonadotropin releasing hormones.
Owner:COUNCIL OF SCI & IND RES
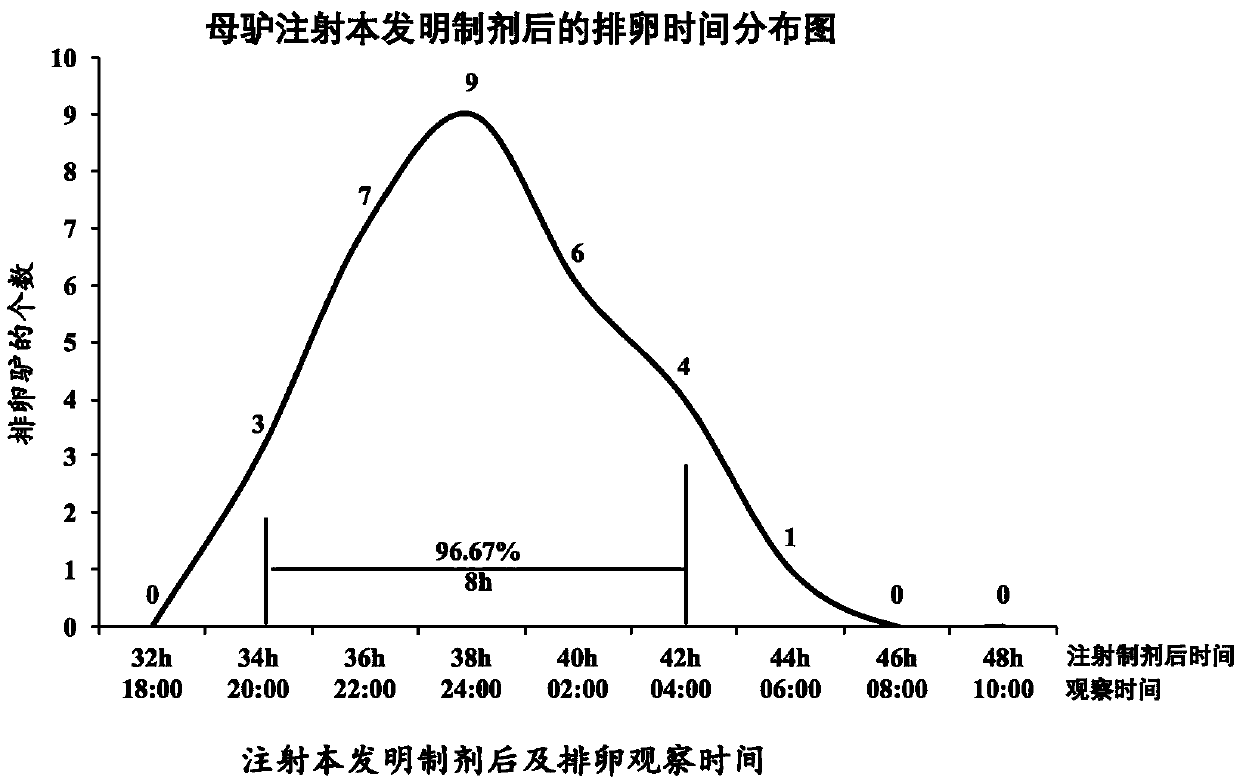
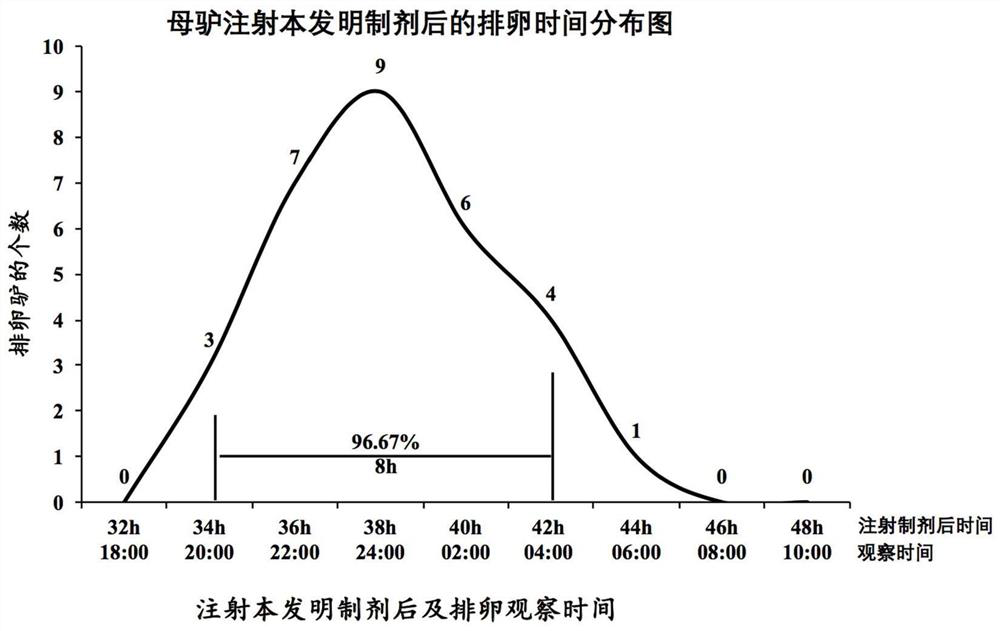
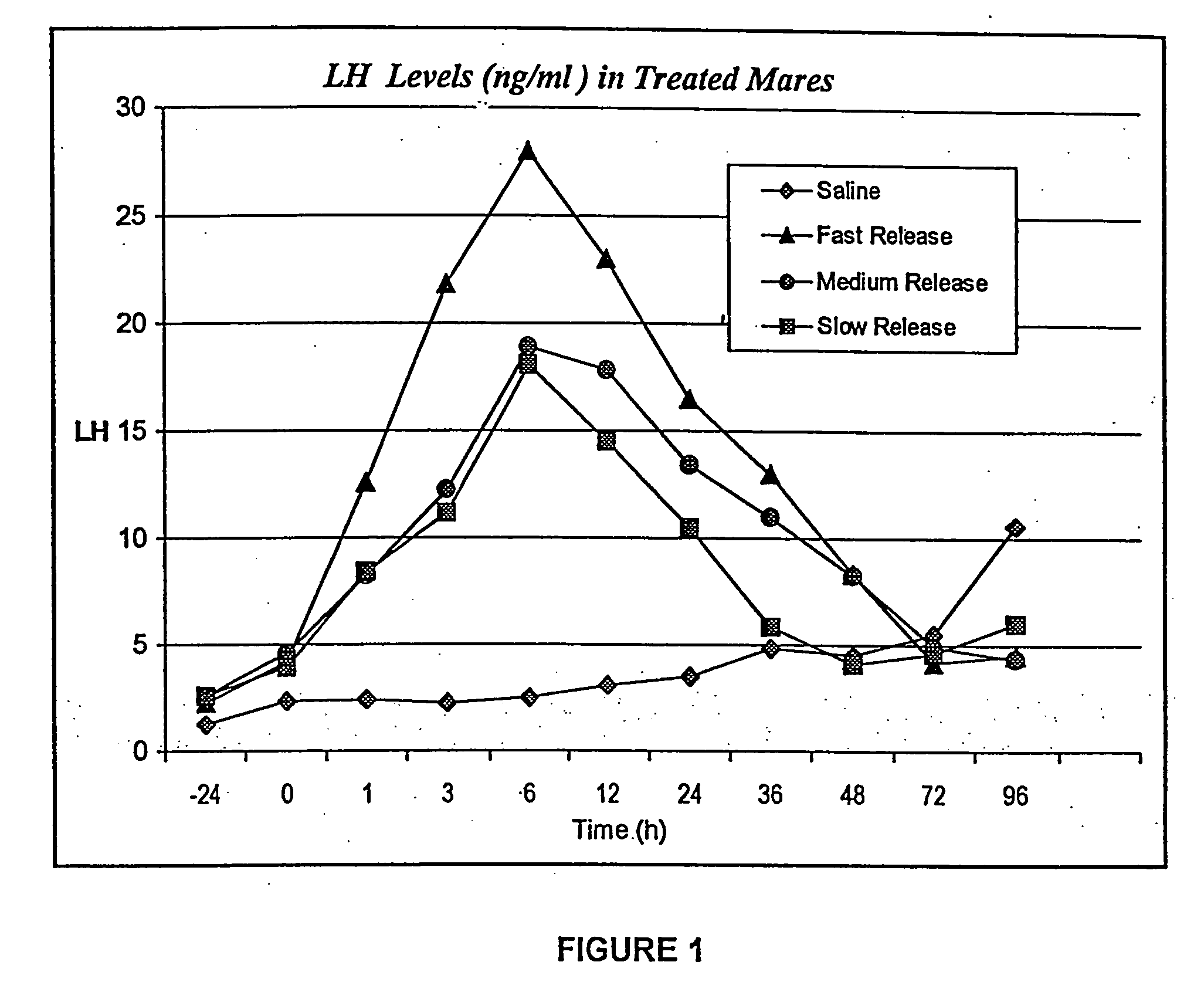
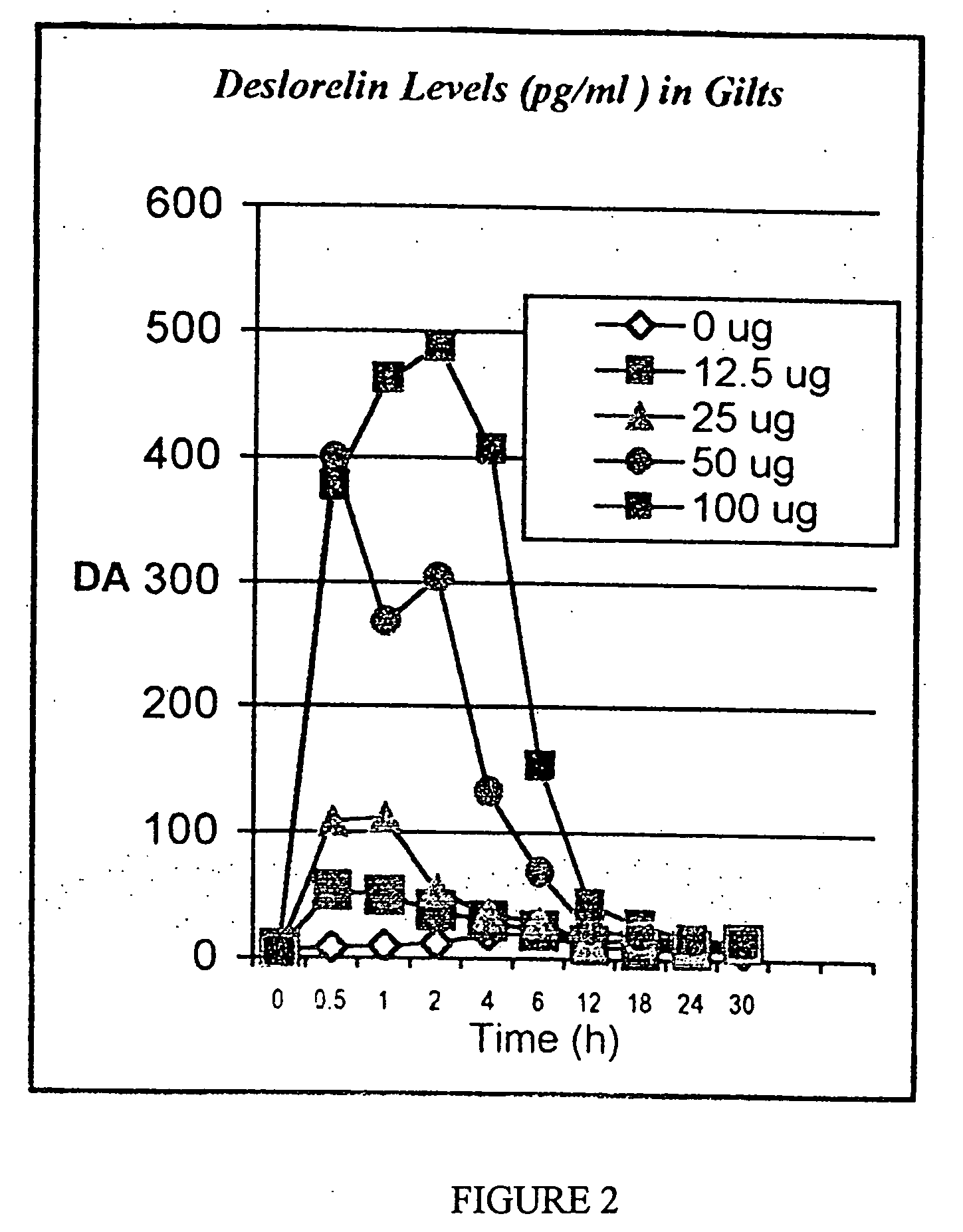
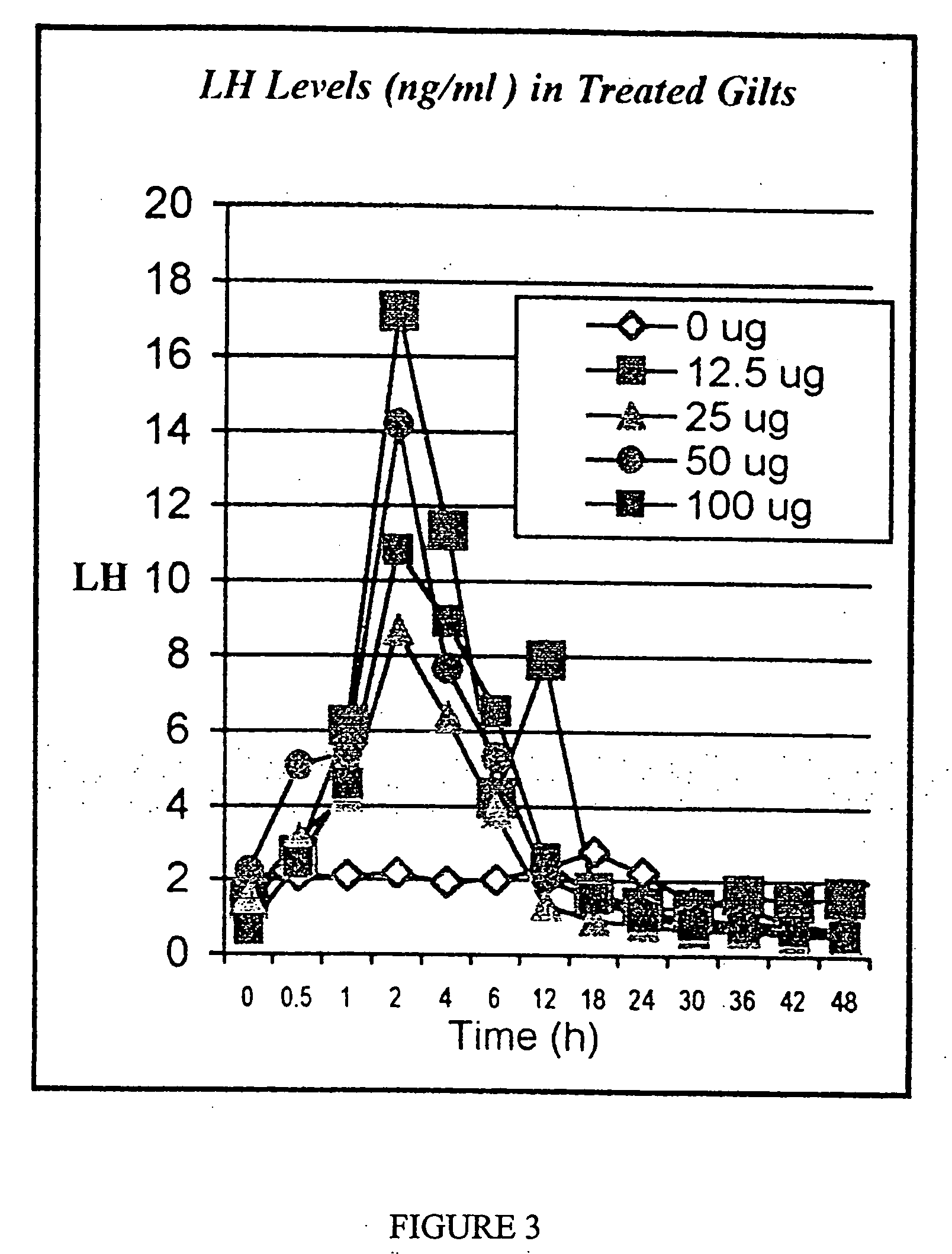
Preparation for controlling ovulation of equus animals and method for controlling ovulation
ActiveCN111202837AImprove pregnancy rateImprove reproductive efficiencyAnimal reproductionPeptide/protein ingredientsAnimal scienceMating
The invention provides a preparation for controlling ovulation of equus animals and a method for controlling ovulation. The preparation comprises a gonadotropin-releasing hormone analogue and a slow release carrier being degradable in bodies of animals. Gonadotropin-releasing hormones are prepared into a slow release preparation, so that the half-life period in bodies can be effectively prolonged,and the preparation can exert the effect of promoting ovulation efficiently and stably for a long time. The preparation disclosed by the invention is injected, 96% or above of female horses and female donkeys can ovulate within 48 hours, and the ovulation time of 96% or above of the animals can be controlled to be within 8 hours, so that efficient ovulation control of the equus animals can be realized, and the breeding pregnancy rate of the equus animals can be notably increased. The ovulation control method is simple to operate, low in cost and notable in control effects, does not cause anyadverse effects on animals and products thereof, can be widely applied to natural mating breeding, artificial insemination, embryo transplanting and timing insemination of the equus animals, and has broad market prospects.
Owner:CHINA AGRI UNIV
Salmonella choleraesuis, vaccine and application thereof
ActiveCN112048459ASuppress aggressionDoes not affect normal growthBacteriaMicroorganism based processesAnimal scienceControl animal
The invention discloses salmonella choleraesuis C500 (pVAX-S / GnRH-2a / KISS1-asd) which is preserved in the China Center for Type Culture Collection and has the preservation number of CCTCC NO:M 2020055. The strain is applied to preparation of drugs or vaccines for controlling animal oestrus performance. The invention further discloses two plasmids, i.e., a pVAX-S / GnRH-asd plasmid and a pVAX-S / GnRH-2a / KISS1-asd plasmid. The two plasmids can also be applied to preparation of the drugs or the vaccines for controlling animal oestrus performance. The invention also discloses a gene vaccine for controlling animal oestrus performance and a preparation method thereof. After animals are immunized by the vaccine, antibodies of a gonadotrophin releasing hormone and KISS1 are generated, animal endogenous hormones are neutralized, aggressivity of the animals is reduced, and the problems of dysphoria, anorexia and abnormal excitation of the animals in the oestrus period are relieved.
Owner:HUAZHONG AGRI UNIV +1
Application of histone methylation H3K4me3 in mouse ovarian development
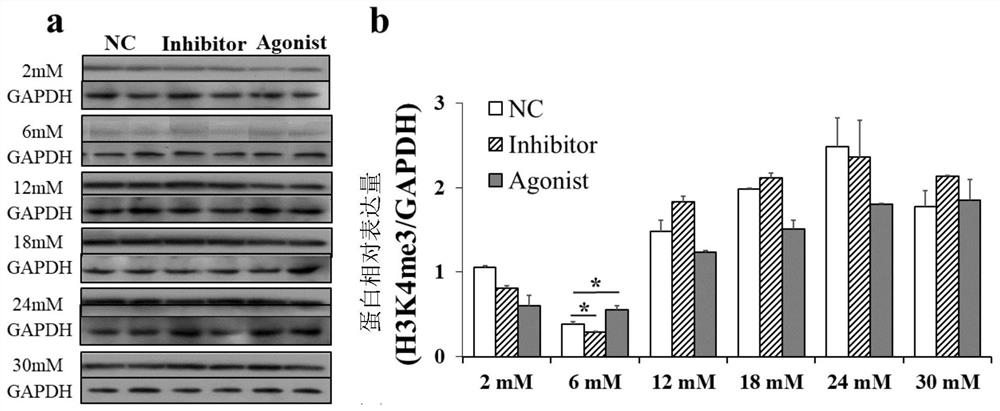
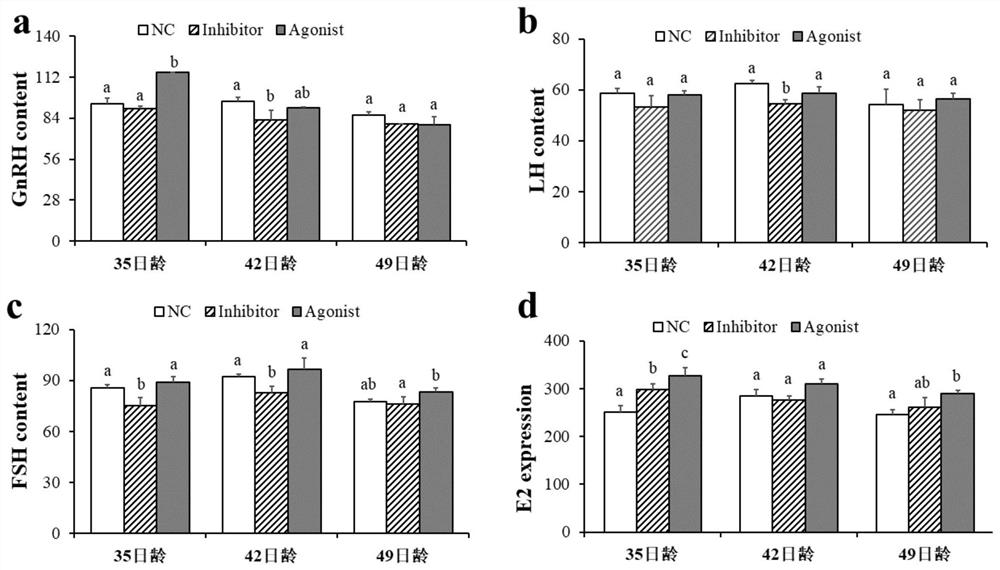
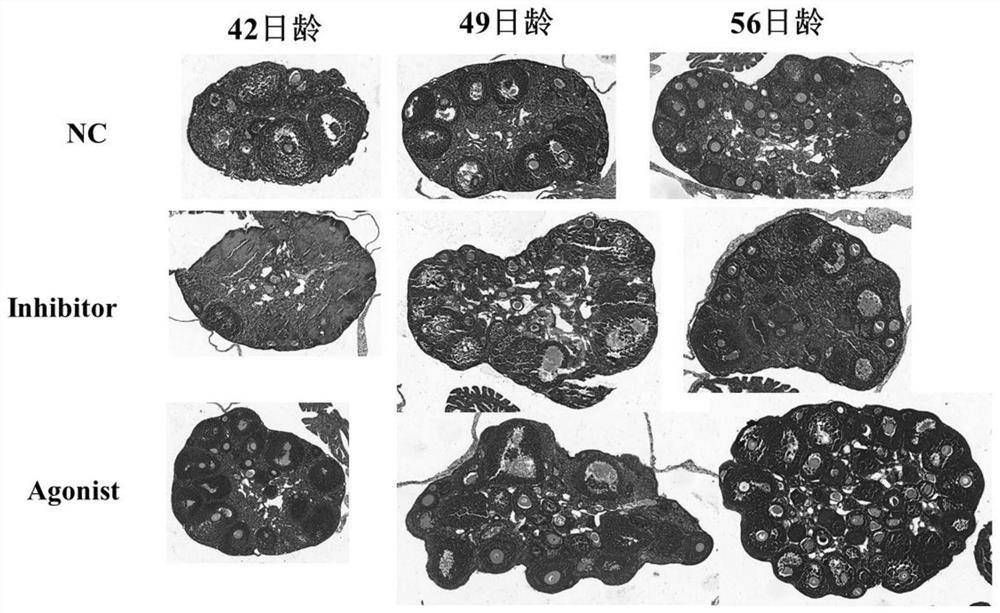
ActiveCN111789966AIncrease litter sizeIncrease litter weightCompounds screening/testingSexual disorderHistone methylationObstetrics
The invention discloses an application of histone methylation H3K4me3 in the mouse ovarian development. The effect of H3K4me3 on the mouse ovarian follicle development is studied from the living bodylevel and protein level, and the result confirms that the H3K4me3 degree increase can promote the mouse ovarian follicle development, inhibit the mouse follicle apoptosis, and increase the litter sizeand litter weight of mice; and the H3K4me3 degree lowering before sexual maturity in mice can inhibit the secretion of GnRH and gonadotropin-releasing hormone LH and FSH in the blood of the mice. A foundation for further exploring the mechanism of H3K4me3 targeting and regulating key genes of the mouse ovarian follicle development at the molecular level, and then regulating the mechanism of the ovarian follicle development is laid.
Owner:SOUTH CHINA AGRI UNIV
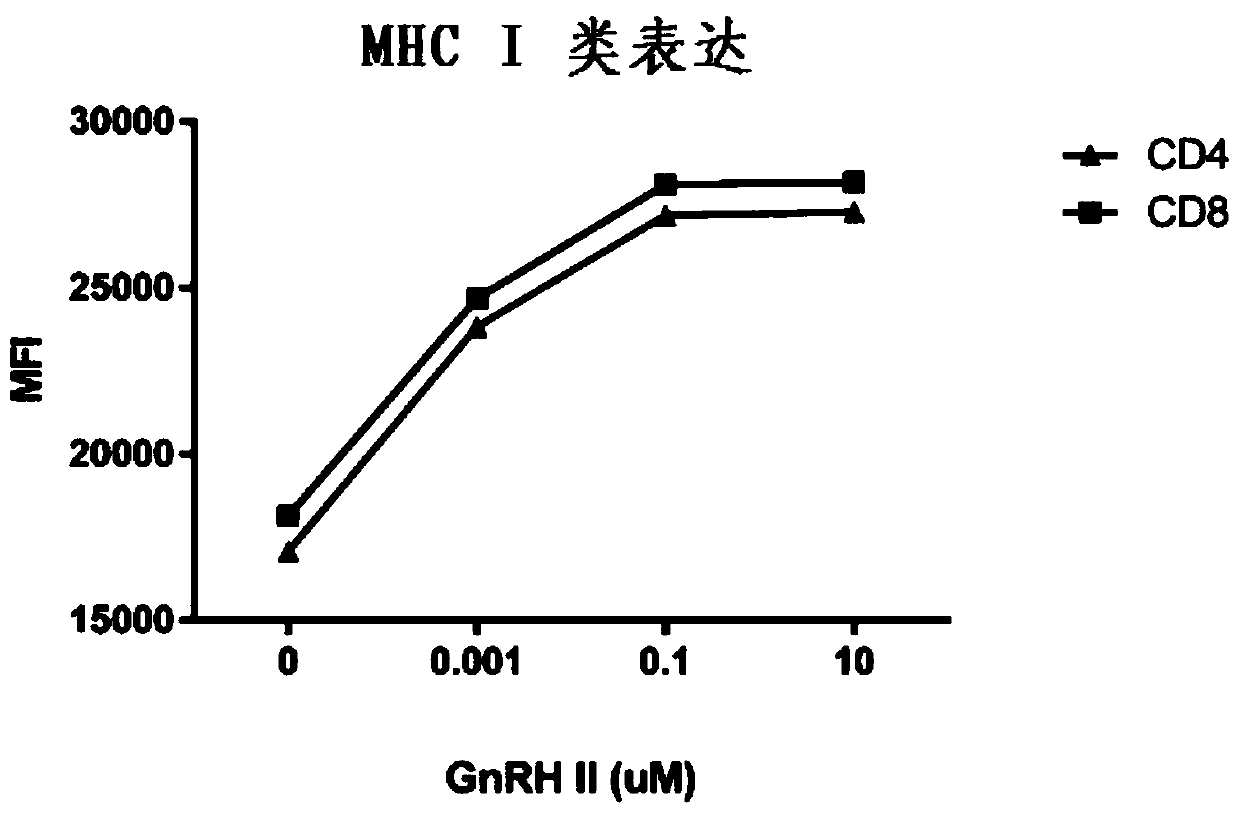
Gonadotropin-releasing hormones for use as adjuvant immunotherapeutics
The present invention relates to the use of a gonadotropin-releasing hormone (including GnRH I, a GnRH I analogue, GnRH II, or a GnRH II analogue) as adjuvant immunotherapeutic.
Owner:ISR IMMUNE SYST REGULATION HLDG AB (PUBL)
Benzooxazole and benzothiazole antagonists of gonadotropin releasing hormone receptor
InactiveUS7531542B2Organic active ingredientsOrganic chemistryGonadotropin-releasing hormone receptorBenzoxazole
The present invention relates to Gonadotropin Releasing Hormone (GnRH, also known as Luteinizing Hormone Releasing Hormone) receptor antagonists.
Owner:WYETH LLC
Products and methods for promoting oestrus in female mammals
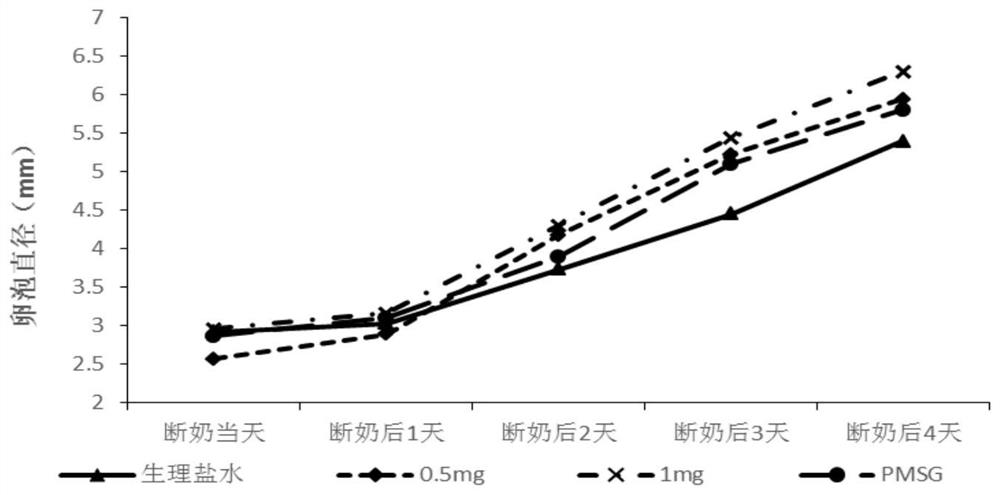
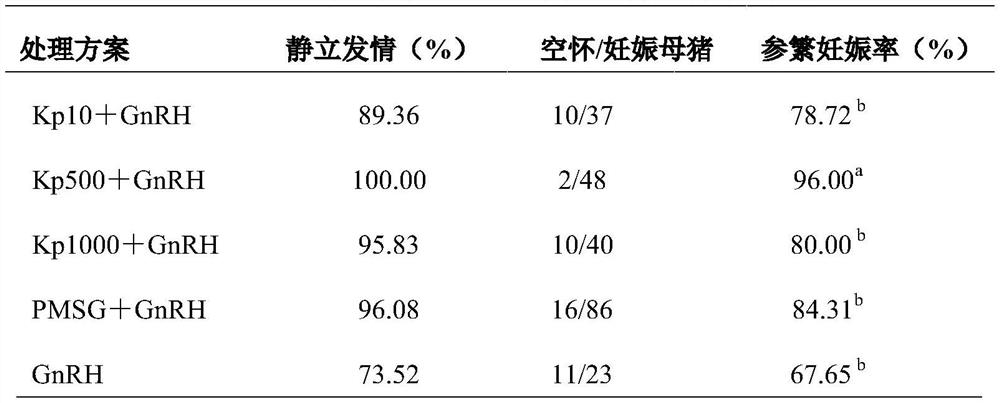
PendingCN114515333APromote follicle developmentIncrease estrus ratePeptide/protein ingredientsSexual disorderMatingMedicine
The invention provides a product for promoting oestrus of female mammals, the product comprises kiss-actuator (Kp) and gonadotropin releasing hormone (GnRH), and oestrus and conception of female animals can be remarkably promoted through the synergistic effect of the kiss-actuator (Kp) and the GnRH. The invention further provides a method for promoting oestrus of the female mammals, an effective dose of Kp is applied in the early oestrus period of the female mammals, then an effective dose of GnRH is applied, follicle development of the female mammals can be effectively promoted, the oestrus rate and conception rate of the female mammals are increased, the missed mating rate of the female mammals with recessive oestrus is reduced, and the oestrus of the female mammals is promoted. The device is suitable for being applied to the synchronous oestrus and timed insemination procedures of the female livestock.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Novel gonadotropin releasing hormone oriented fusion protein mutant
The invention relates to a fusion protein mutant capable of directionally killing tumour cells, which consists of an orienting part and an effect part, wherein the orienting part consists of gonadotropin releasing hormone (GnRH), the effect part consists of cytotoxin, and the fusion protein mutant has the characteristics that the tumour cells excessively expressing GnRH receptors can be directionally reached and killed.
Owner:BEIJING YIDE XINAO BIOLOGICAL TECH
COMPOSITIONS AND METHODS FOR LONG TERM RELEASE OF GONADOTROPIN-RELEASING HORMONE (GnRH) ANTAGONISTS
PendingUS20200282008A1Achieve effectGood effectPeptide/protein ingredientsAerosol deliveryGonadotropin-releasing hormone antagonistControl release
The invention provides compositions and methods for long term release of gonadotropin-releasing hormone (GnRH) antagonists and uses thereof. Specifically, the invention provides polymer compositions and methods for controlled release of GnRH antagonists.
Owner:VERU INC
Recombinant target fusion protein GnRH-TNFam and its antitumor use
The invention provides a novel recombination targeting fusion protein GnRH-TNF alpham which is integrated from gonadotrophin releasing hormone (GnRH-A) and recombinant mutant human tumor necrosis factor alpha (TNF alpha), the targeting fusion protein has destroying action to the tumor cells endured by the prototype TNF alpha. The invention also provides the DNA sequence of the recombinant target fusion protein GnRH-TNFam and its expression recombinants.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
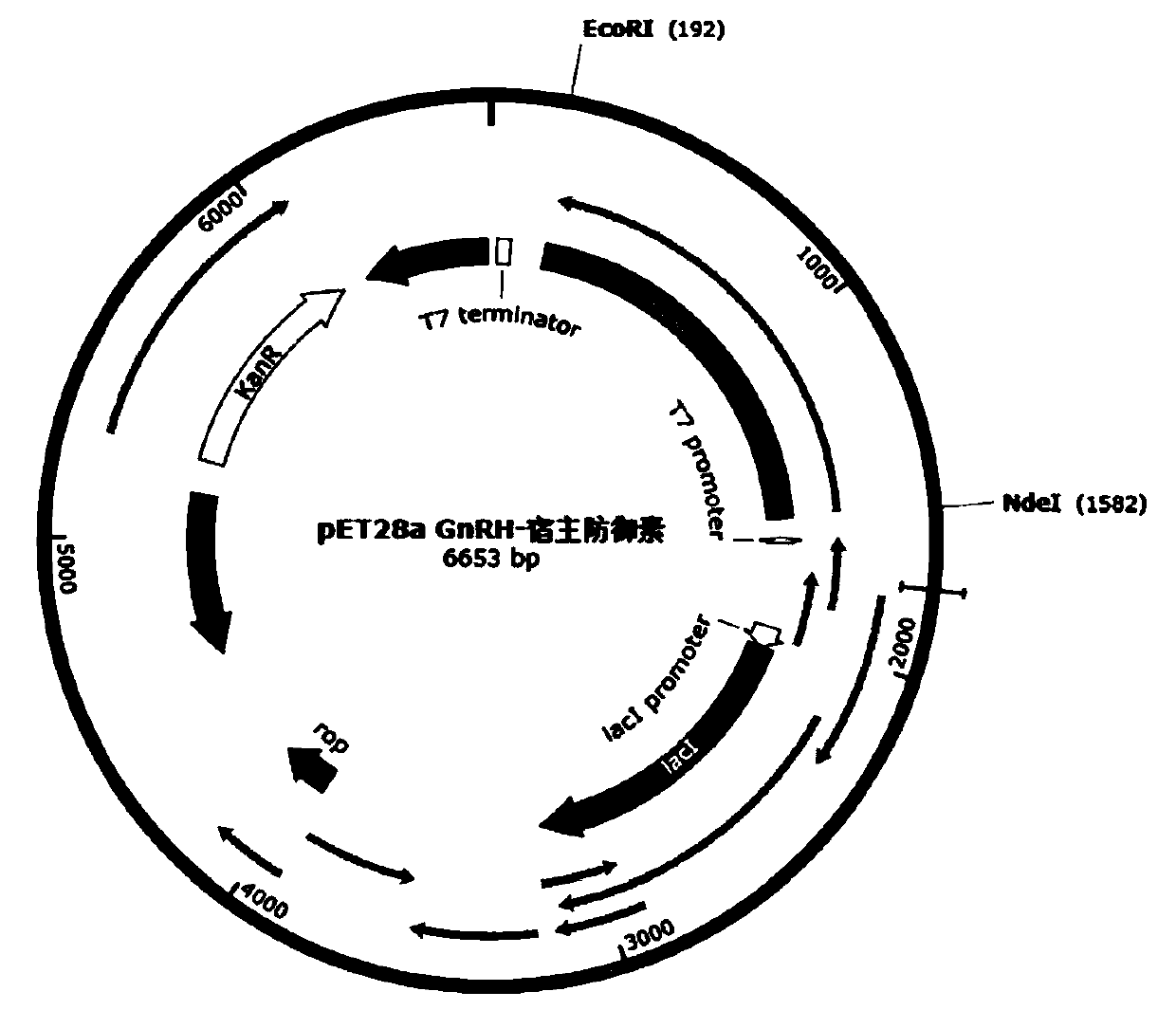
gnrh-defensin recombinant castration vaccine and its preparation
ActiveCN108904788BFertility suppressionAntibody mimetics/scaffoldsLuteinising hormone-releasing hormoneHeterologousPhysiology
The invention belongs to the technical field of the molecular biology and describes a fusion vaccine containing an encoded immunocontraceptive protein gonadotropin-releasing hormone (GnRH) and a heterologous defensin polypeptide (HDP). The invention discloses a method for construction, transformation expression and purification of the recombinant GnRH-HDP fusion protein vaccine. Through inoculation of animals, a contraceptive peptide-specific antibody is produced through immunization and controls the animal fertility. The GnRH-HDP defensin immunogenic composition can effectively control the fertility of the animals.
Owner:广州源博医药科技有限公司
A preparation for controlling ovulation of equine animals and a method for controlling ovulation
ActiveCN111202837BImprove pregnancy rateImprove reproductive efficiencyAnimal reproductionPeptide/protein ingredientsAnimal scienceEmbryo
The invention provides a preparation for controlling ovulation of equine animals and a method for controlling ovulation. The preparation comprises a gonadotropin-releasing hormone analogue and a degradable slow-release carrier in the animal body. By preparing the gonadotropin-releasing hormone as a slow-release preparation, its half-life in the body is effectively prolonged, so that the preparation can play a long-term, efficient and stable role in promoting ovulation. By injecting the preparation of the present invention, more than 96% of mares and donkeys ovulate within 48 hours, and the ovulation time of more than 96% of the animals is controlled within 8 hours, realizing efficient ovulation control of equine animals and significantly improving Breeding pregnancy rates in equines. The ovulation control method of the present invention is easy to operate, low in cost and remarkable in control effect, does not produce any harmful effects on the animal itself and its products, and can be widely used in mating species of equine animals, artificial insemination, embryo transfer and timed insemination, Has broad market prospects.
Owner:CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/b283e538-3f46-4a03-bf80-a554d8c2900a/US20060189617A1-20060824-C00001.png)
![Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/b283e538-3f46-4a03-bf80-a554d8c2900a/US20060189617A1-20060824-C00002.png)
![Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/b283e538-3f46-4a03-bf80-a554d8c2900a/US20060189617A1-20060824-C00003.png)

![Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/0928bf2c-5b7a-464d-a1f5-c15fce86653f/US07534796-20090519-C00001.png)
![Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/0928bf2c-5b7a-464d-a1f5-c15fce86653f/US07534796-20090519-C00002.png)
![Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/0928bf2c-5b7a-464d-a1f5-c15fce86653f/US07534796-20090519-C00003.png)