Drug sustained release microsphere, preparation method and application thereof
A technology of slow-release microspheres and drugs, which is applied in drug combination, drug delivery, and pharmaceutical formulations. Solve the effect of poor repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
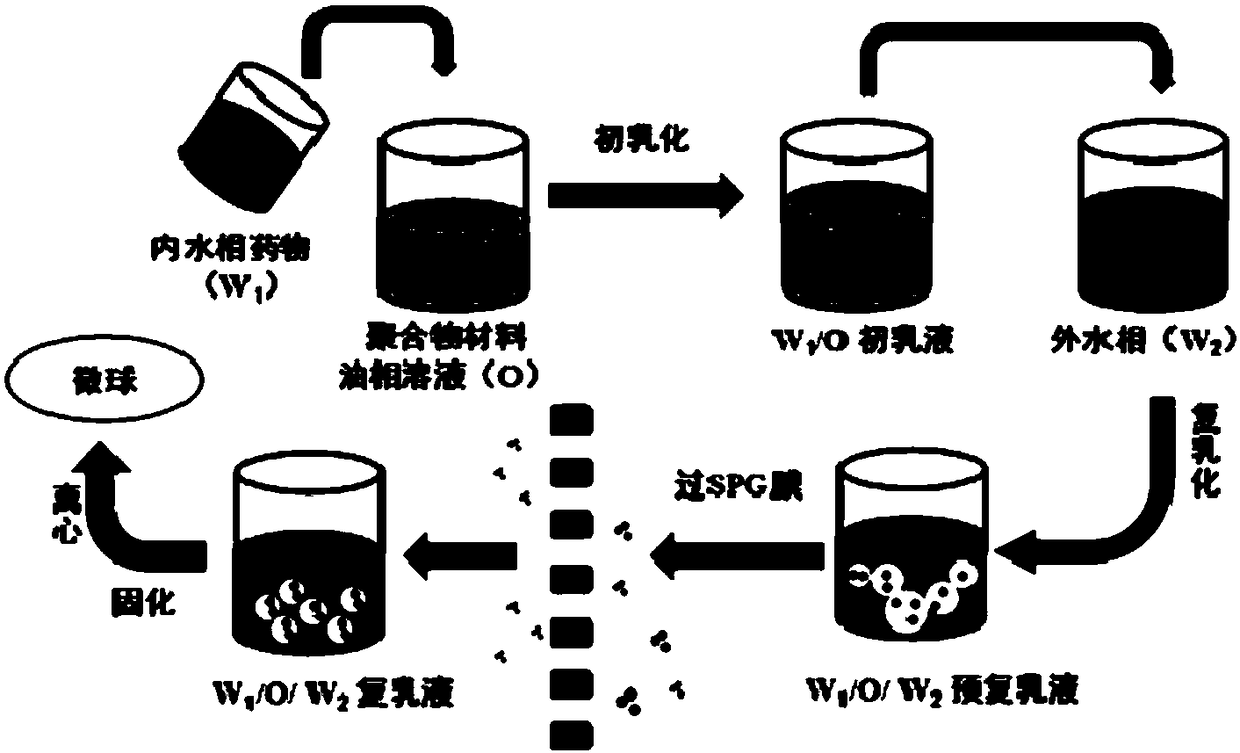
[0069] A preparation method for embedding triptorelin acetate long-acting sustained-release microspheres for injection, comprising the following steps (the schematic diagram of its preparation process is as follows: figure 1 shown):
[0070] The hydrophilic microporous membrane with a pore size of 80 μm is soaked in water to fully wet the porous membrane. 20mL concentration is 50mg / mL triptorelin aqueous solution as inner water phase W 1 , Dissolve 1 g of polylactic-co-glycolic acid (PLGA) with a molecular weight of 30,000 in 20 mL of acetone as oil phase O. Mix the inner water phase and the oil phase, homogeneously emulsify for 1 min, and get W 1 / O colostrum. The colostrum was added to 200 mL of 5% wt PVA aqueous solution W 2 , magnetic stirring at 600rpm for 1min to prepare W 1 / O / W 2 type pre-double emulsion, and then this pre-double emulsion is pressed through the microporous membrane device (such as figure 1 SPG film in ), get W 1 / O / W 2Type double emulsion, and...
Embodiment 2
[0081] A preparation method for embedding leuprolide long-acting sustained-release microspheres for injection, comprising the following steps (the schematic diagram of its preparation process is as follows: figure 1 shown):
[0082] The hydrophilic microporous membrane with a pore size of 32 μm was soaked in water to fully wet the porous membrane. 5 mL of 20 mg / mL leuprolide aqueous solution was used as the internal water phase, and 100 mg of polylactic-co-glycolic acid (PLGA) with a molecular weight of 10,000 was dissolved in 10 mL of ethyl acetate as the oil phase. Mix the inner water phase and the oil phase, homogeneously emulsify for 1 min, and get W 1 / O colostrum. The colostrum was added to 200 mL of 1% wt PVA aqueous solution W 2 , magnetically stirred at 500rpm for 2min to prepare W 1 / O / W 2 type pre-double emulsion, and then this pre-double emulsion is pressed through the microporous membrane device (such as figure 1 SPG film in ), get W 1 / O / W 2 Then the doub...
Embodiment 3
[0092] A preparation method for embedding leuprolide long-acting sustained-release microspheres for injection, comprising the following steps (the schematic diagram of its preparation process is as follows: figure 1 shown):
[0093] The hydrophilic microporous membrane with a pore size of 60 μm is soaked in water to fully wet the porous membrane. 10mL concentration of 10mg / mL leuprolide aqueous solution as the inner water phase W 1 , 100 mg of polylactic acid (PLA) with a molecular weight of 10,000 was dissolved in 10 mL of ethyl acetate as oil phase O. Mix the inner water phase and the oil phase, homogeneously emulsify for 30s, and get W 1 / O colostrum. This colostrum was added to 100 mL of 5% wt PVA aqueous solution W 2 In, magnetic stirring 300rpm stirs 30s to prepare pre-multiple emulsion, then this pre-multiplex emulsion is pressed through the microporous membrane device (such as figure 1 SPG film in ), get W 1 / O / W 2 Then the double emulsion was solidified at a va...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com