Treatment of trop-2 expressing triple negative breast cancer with sacituzumab govitecan and a rad51 inhibitor
一种抑制剂、拓扑替康的技术,应用在用沙妥珠单抗格维替康和RAD51抑制剂治疗表达TROP-2的三阴性乳腺癌领域,能够解决延迟、剂量减少等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0213] Example 1. Targeted therapy of gastrointestinal cancer with IMMU-132 (sartuzumab gvetecan, an anti-Trop-2-SN-38 antibody drug conjugate (ADC))
[0214] Trop-2 is a very prevalent tumor-associated glycoprotein in many epithelial cancers. Elevated expression has been associated with more aggressive disease and poor prognosis. A humanized mAb that binds the extracellular domain of Trop-2 was conjugated to SN-38 (IMMU-132; average drug:mAb ratio = 7.6), which is the active ingredient of CPT-11. Following potent activity in human tumor xenografts, phase I / II trials were initiated in patients (pts) with different solid tumors, including gastrointestinal cancers.
[0215] method : Patients with metastatic cancer were recruited after failure of standard therapy to start at a dose of 8.0 mg / kg given on days 1 and 8 of a 3-week cycle. The MTD was determined to be 12 mg / kg; dose levels of 8 and 10 mg / kg were selected for Phase II trials.
[0216] result: Sixty patients with...
Embodiment 2
[0219] Example 2. Production and use of anti-Trop-2-SN-38 antibody-drug conjugates
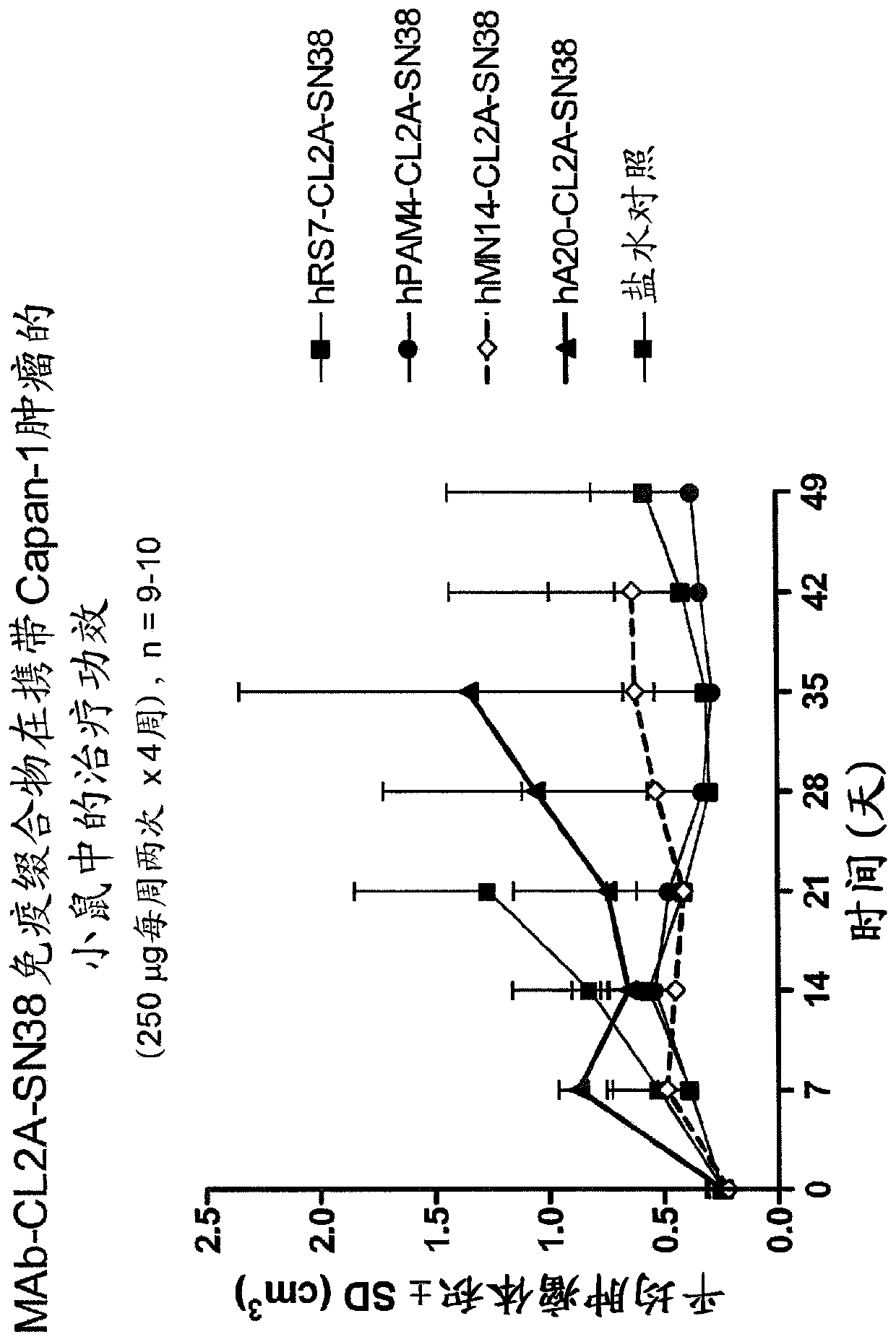
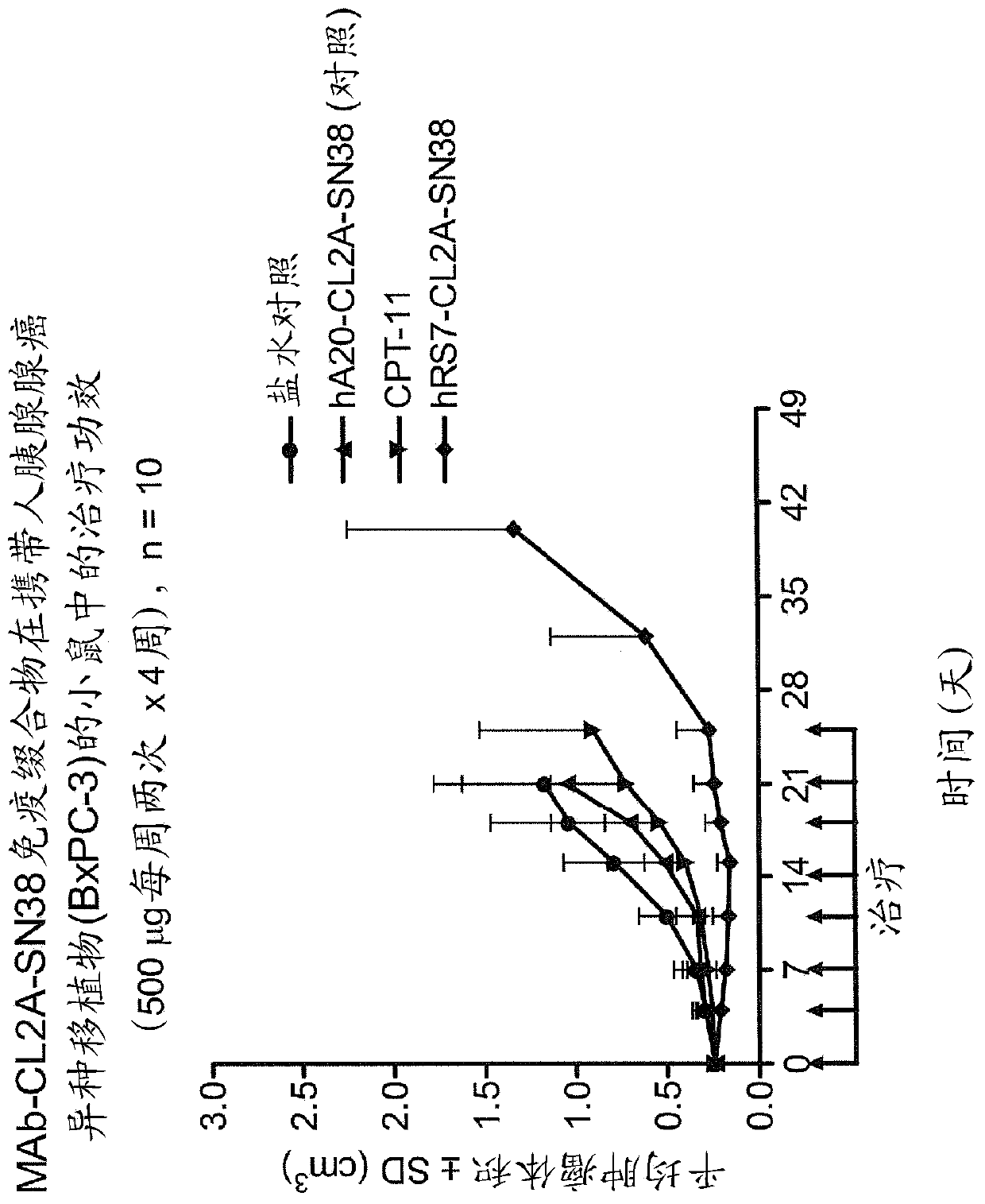
[0220] Humanized RS7 (hRS7) anti-Trop-2 antibodies were generated as described in US Patent No. 7,238,785, the Figures and Examples section of which are incorporated herein by reference. According to U.S. Patent 7,999,083 (Examples 10 and 12 of which are incorporated herein by reference), SN-38 attached to a CL2A linker was generated and combined with hRS7 (anti-Trop-2), hPAM4 (anti-MUC5ac), hA20 (anti- -CD20) or hMN-14 (anti-CEACAM5) antibody conjugates. The conjugation protocol yielded a ratio of about 6 SN-38 molecules attached per antibody molecule.
[0221] Immunocompromised athymic nude mice (female) bearing subcutaneous human pancreatic or colon tumor xenografts were treated or not with specific CL2A-SN-38 conjugates or control conjugates. The therapeutic efficacy of the specific conjugates was observed. figure 1shows the Capan 1 pancreatic tumor model in which specific CL2A-SN-38 co...
Embodiment 3
[0222] Example 3. Efficacy of anti-Trop-2-SN-38 ADC against different epithelial cancers in vivo
[0223] Summary
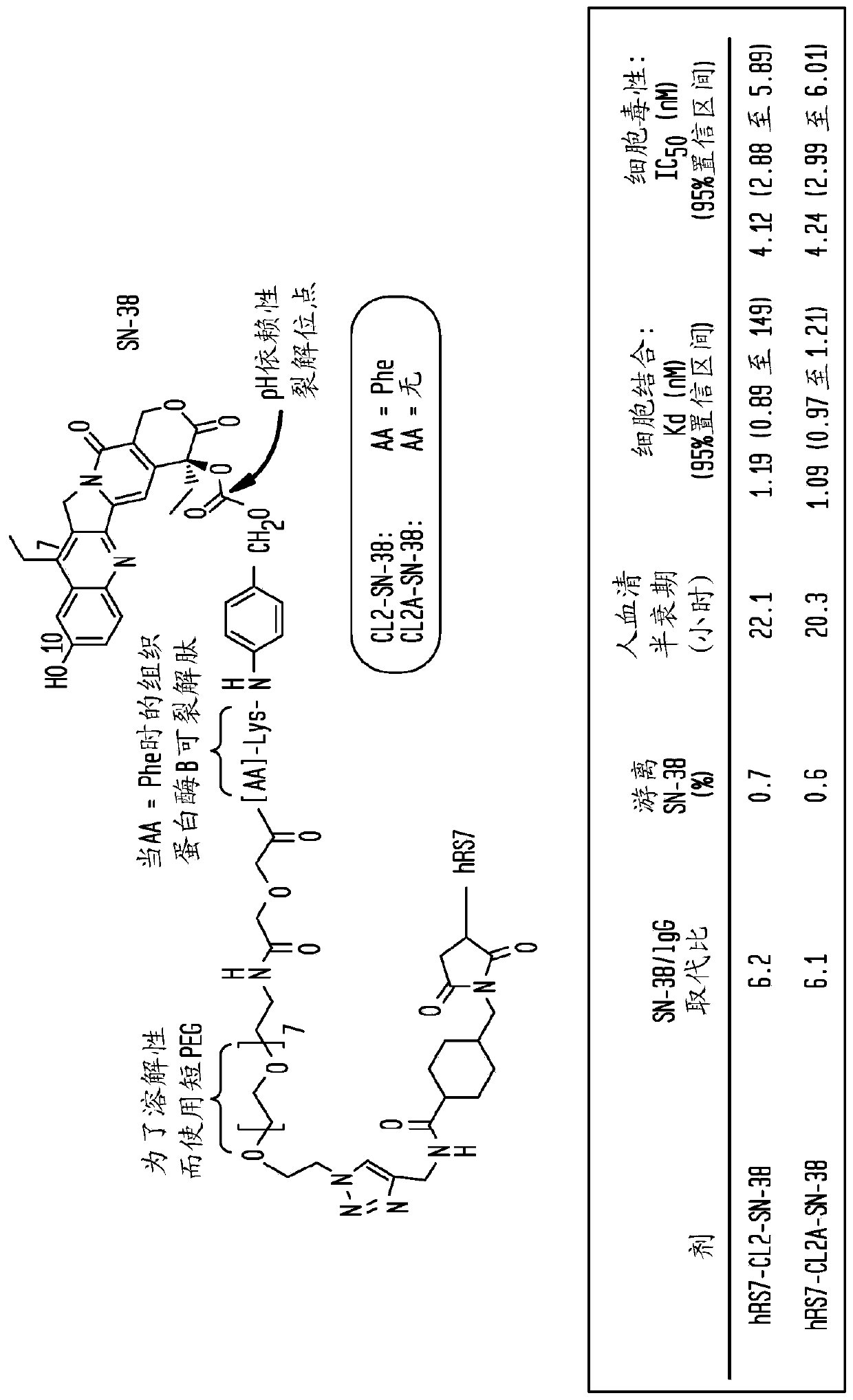
[0224] The purpose of this study was to evaluate the efficacy of the SN-38-anti-Trop-2(hRS7) ADC against several human solid tumor types and to assess its tolerability in mice and monkeys, which are similar to humans and have the same Tissue cross-reactivity. Two SN-38 derivatives, CL2-SN-38 and CL2A-SN-38, were conjugated to the anti-Trop-2 humanized antibody hRS7. Stability, binding and cytotoxicity of the immunoconjugates were characterized in vitro. Efficacy was tested in five different human solid tumor-xenograft models expressing the Trop-2 antigen. Toxicity was assessed in mice and cynomolgus monkeys.
[0225] The hRS7 conjugates of the two SN-38 derivatives were highly effective in drug substitution (~6), cell binding (K d ~1.2nmol / L), cytotoxicity (IC 50 ~2.2nmol / L) and in vitro serum stability (t / 1 / 2 ~20 hours) are equivalent. Exposure of cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com