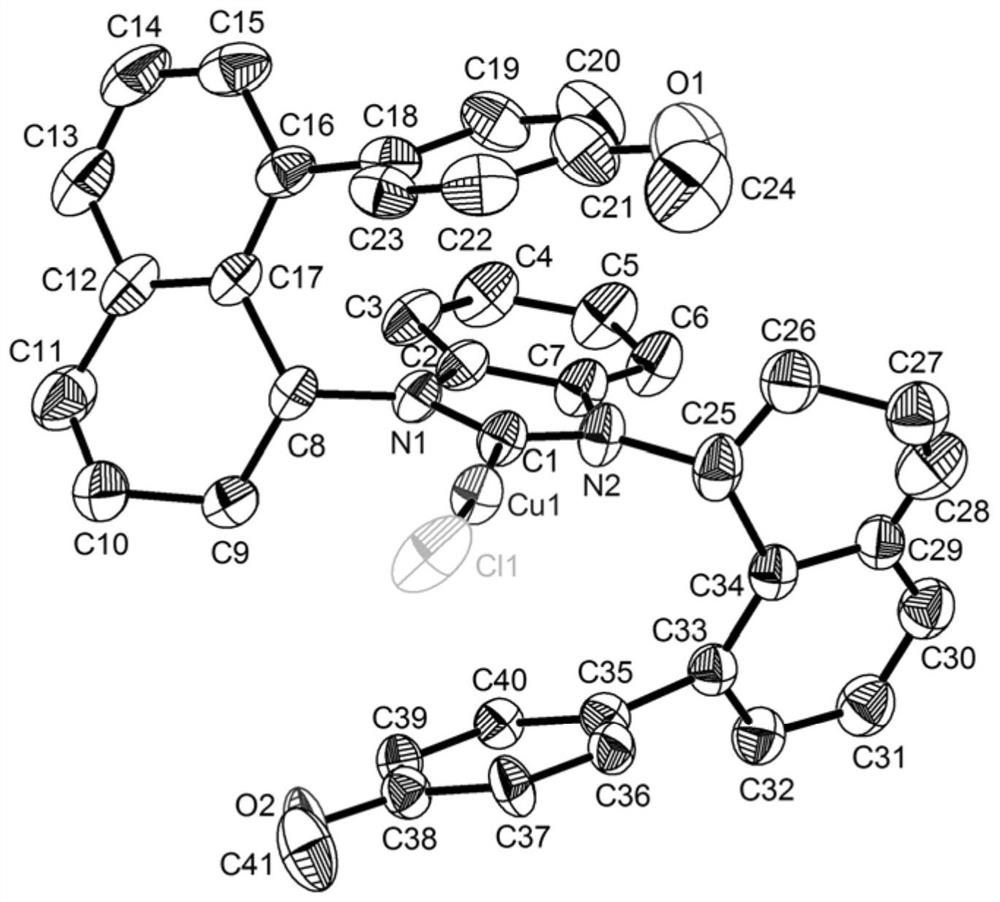
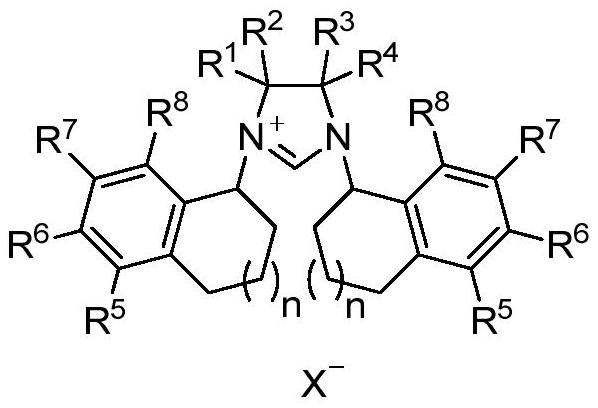
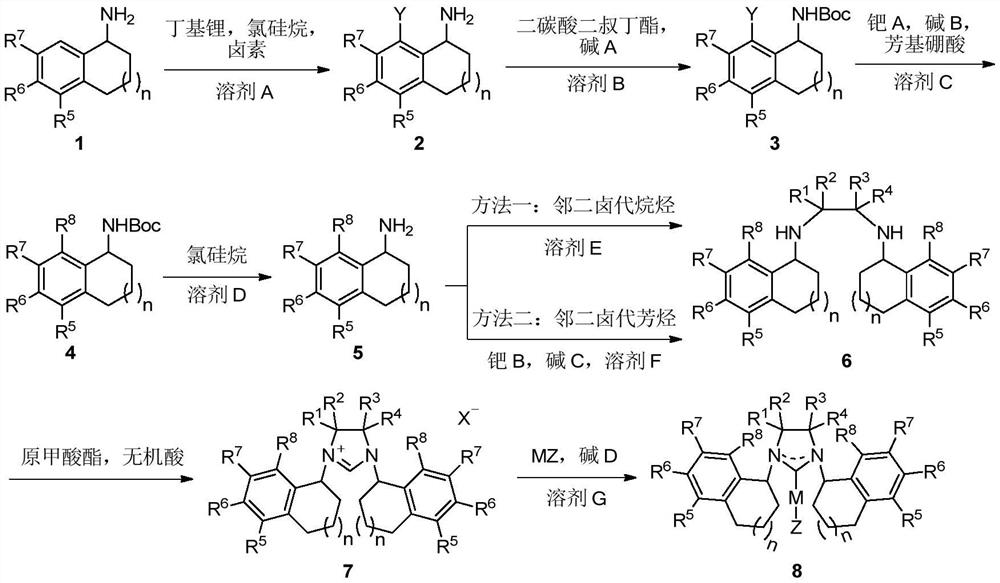
A class of chiral carbene precursor compounds with sandwich structure and synthesis method thereof
A precursor compound and sandwich structure technology, which is applied to a class of chiral carbene precursor compounds with a sandwich structure and the field of synthesis thereof, and can solve the problem of decreased carbene stability, difficulty in obtaining metal-carbene complexes, and limiting sandwich structure carbene Ligand application and other issues, to achieve the effect of asymmetric hydrosilylation, novel structure and fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The synthesis of embodiment 1 compound (R)-2
[0051]
[0052]Under nitrogen atmosphere, (R)-tetrahydronaphthylamine (R)-1 (7.36 g, 50 mmol) was dissolved in 80 mL tetrahydrofuran in a 250 mL reaction flask. At -78°C, n-butyl lithium (34.3 mL, c=1.6 mol / L, 55 mmol) was added dropwise to the reaction solution, and stirred for 30 minutes. At this temperature, a tetrahydrofuran solution (40 mL) of tert-butyldimethylsilyl chloride (8.29 g, 55 mmol) was added dropwise, and stirred overnight after naturally returning to room temperature. After stopping the reaction, the solvent was removed under reduced pressure and used directly in subsequent reactions. Under nitrogen atmosphere, the above crude product was dissolved in 150 mL of ether in a 500 mL reaction flask. At -78°C, n-butyllithium (93.8 mL, c=1.6 mol / L, 150 mmol) was added dropwise to the reaction solution, and the reaction solution returned to room temperature within 3 hours, and continued to stir for 1 hour. T...
Embodiment 2
[0053] The synthesis of embodiment 2 compound (R)-3
[0054]
[0055] In a 250 mL reaction flask, the iodo substrate (R)-2 (5.46 g, 20 mmol) and triethylamine (3.04 g, 30 mmol) were dissolved in 50 mL of dichloromethane. At 0°C, a dichloromethane solution (50 mL) of di-tert-butyl dicarbonate (5.24 g, 24 mmol) was added dropwise into the reaction system, and stirred overnight at room temperature. After stopping the reaction, 50 mL of water was added to the reaction liquid, extracted with dichloromethane, the organic phases were combined, and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure and separated by column chromatography to obtain 6.94 g of a yellow viscous liquid with a yield of 93%. 1 H NMR (400MHz, CDCl 3 )δ7.72(d, J=7.7Hz, 1H), 7.07(d, J=7.6Hz, 1H), 6.87(t, J=7.7Hz, 1H), 4.73-4.17(m, 2H), 2.80- 2.65(m,2H),2.22(d,J=11.9Hz,1H),1.78-1.62(m,3H),1.46(s,9H). 13 C NMR (100MHz, CDCl 3 )δ154.8, 140.5, 138.0, 129.6, 129.2, 103.2, 79.3...
Embodiment 3
[0056] The synthesis of embodiment 3 compound (R)-4
[0057]
[0058] Under nitrogen atmosphere, in 250mL Schlenk bottle, add substrate (R)-3 (6.72g, 18mmol), 4-methoxyphenylboronic acid (4.10g, 27mmol), tetrakis (triphenylphosphine) palladium (1.04g, 0.9mmol), 2mol / L potassium carbonate (4.98g, 36mmol) aqueous solution (18mL) and ethylene glycol dimethyl ether (180mL), the reaction solution was heated to 85°C and refluxed for 36 hours. The reaction system was cooled to room temperature, then poured into 50 mL of water, extracted with ethyl acetate, combined the organic phases, and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure and separated by column chromatography to obtain the crude coupling target product, which could be directly used in subsequent reactions without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com