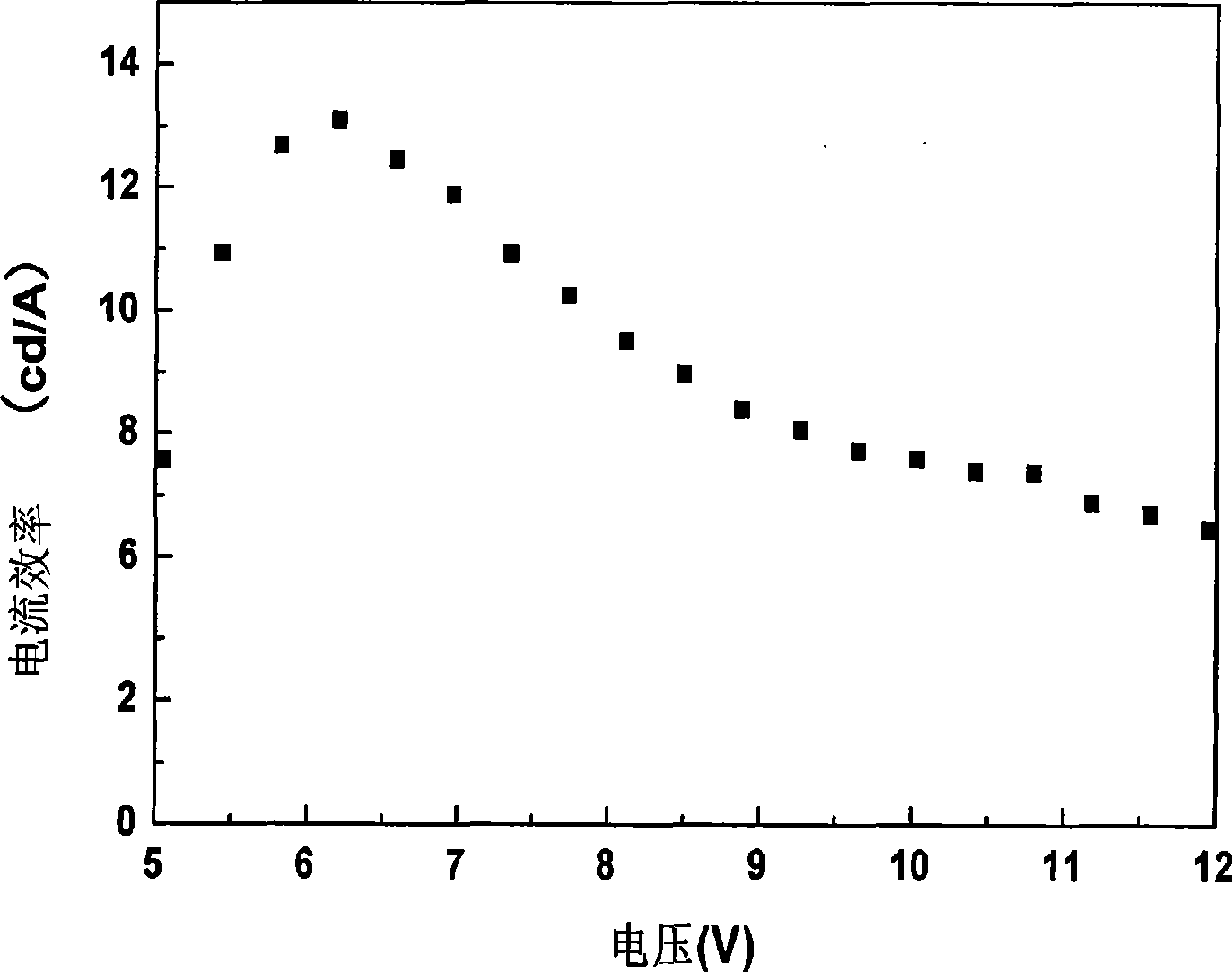
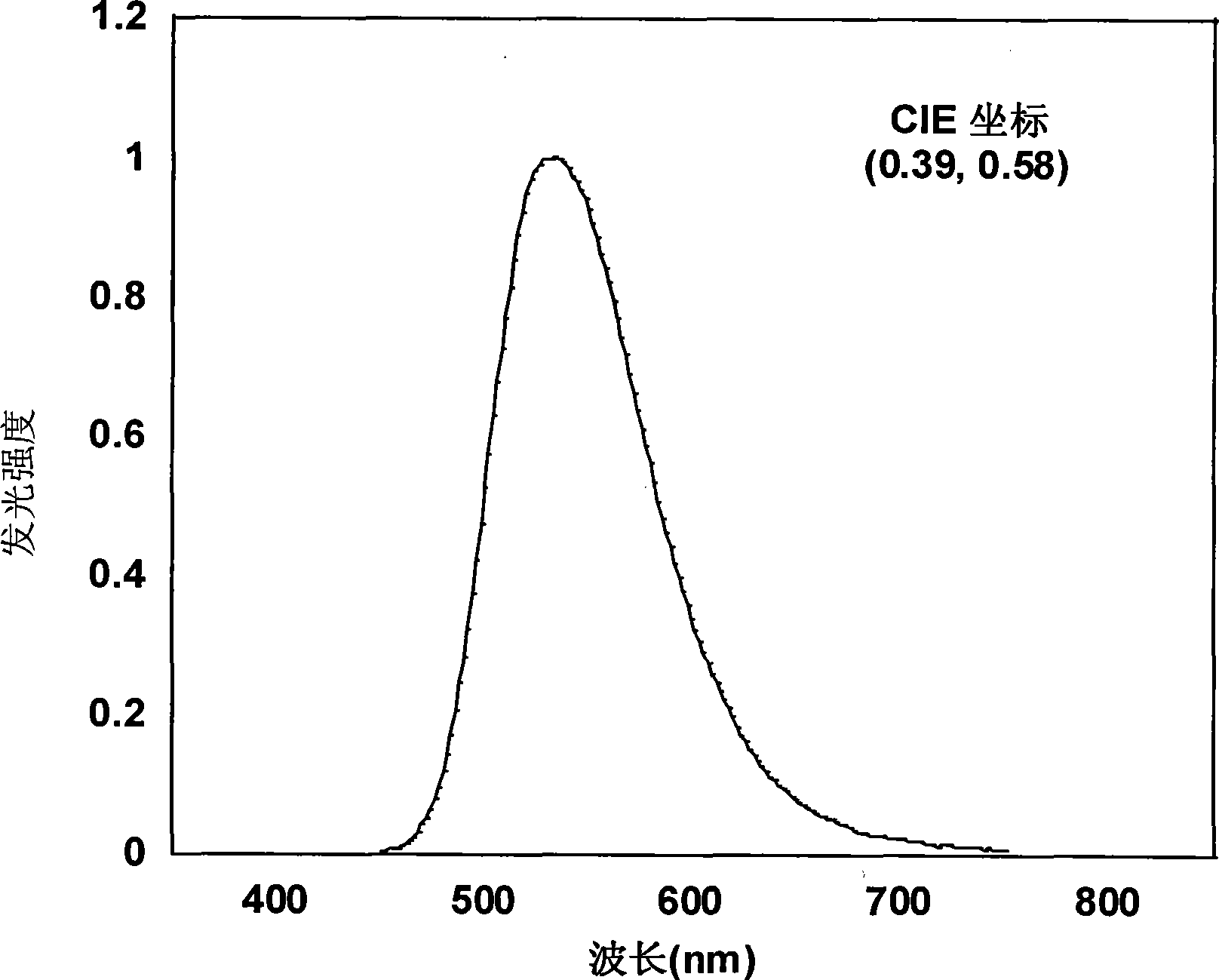
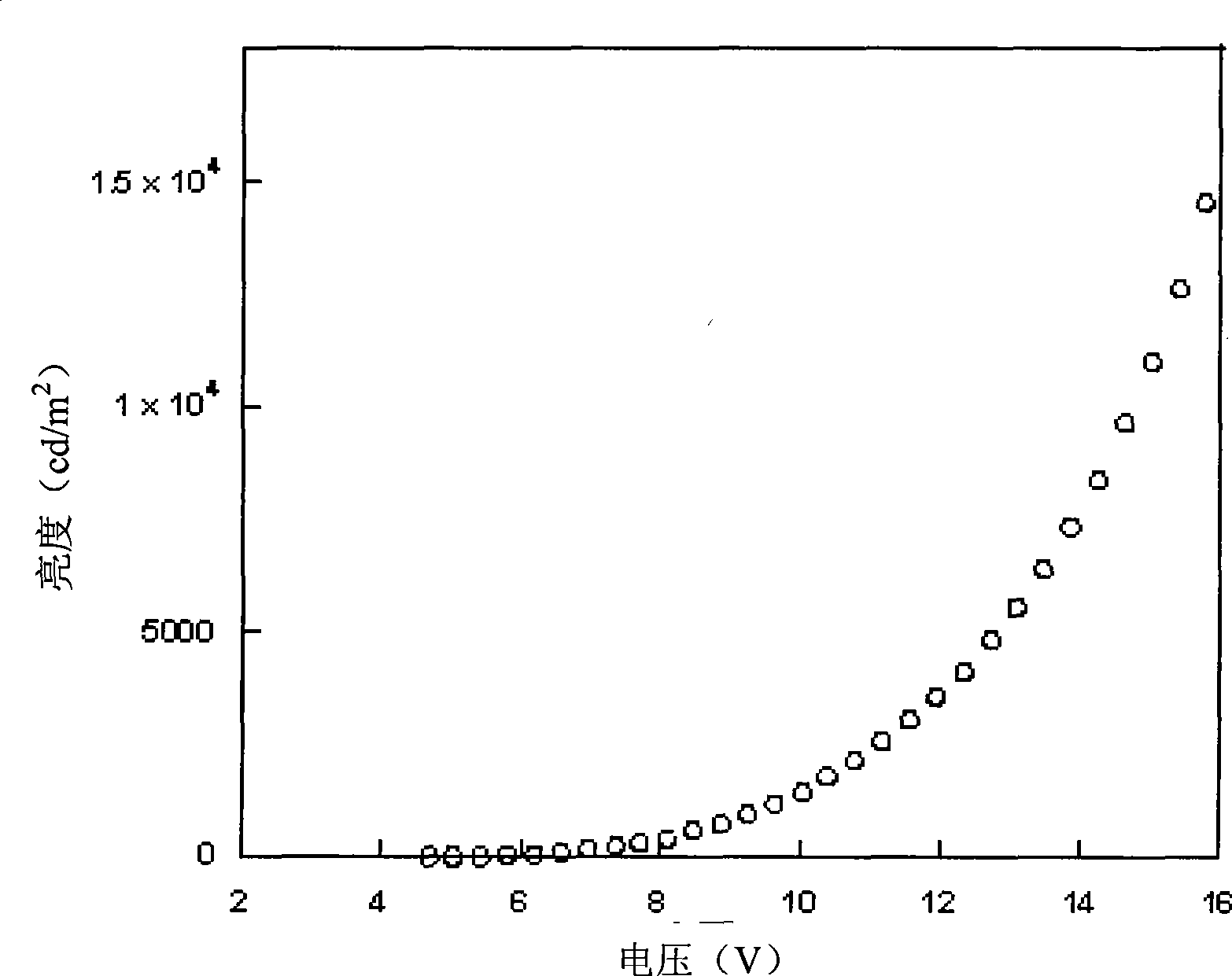
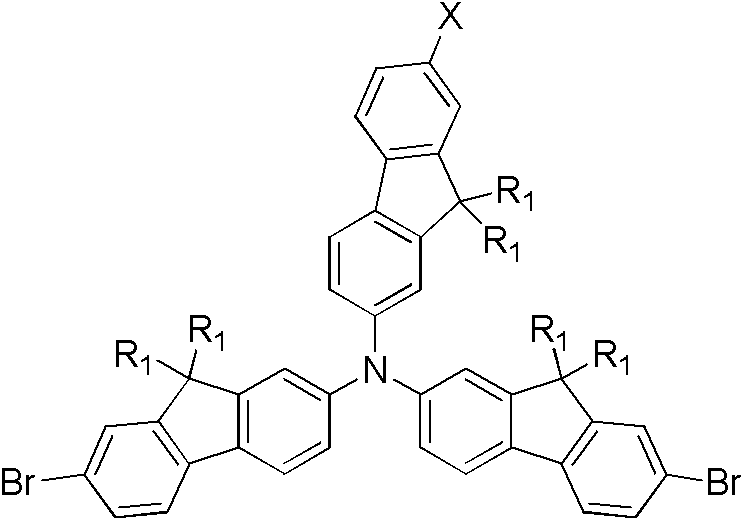
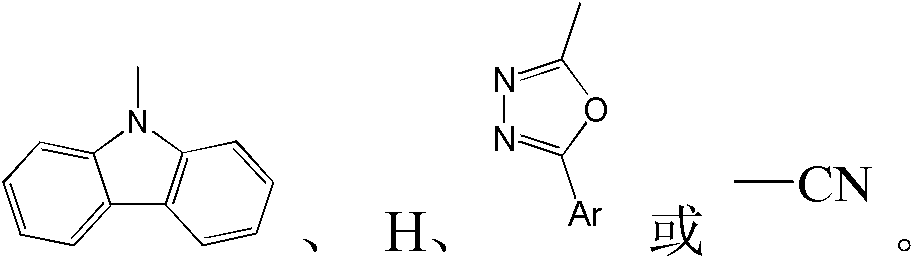
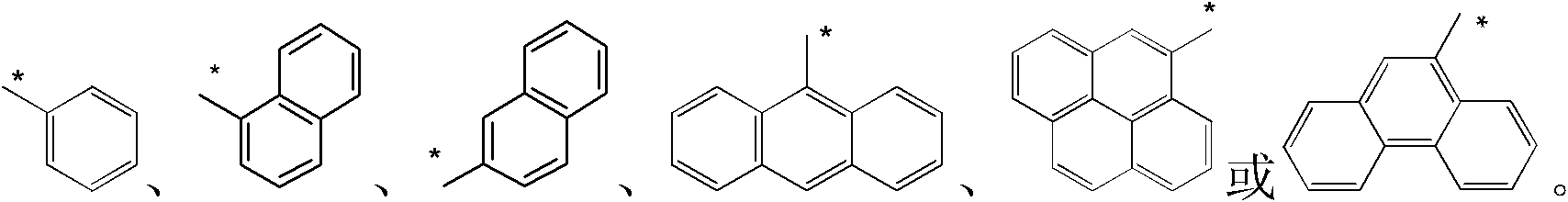
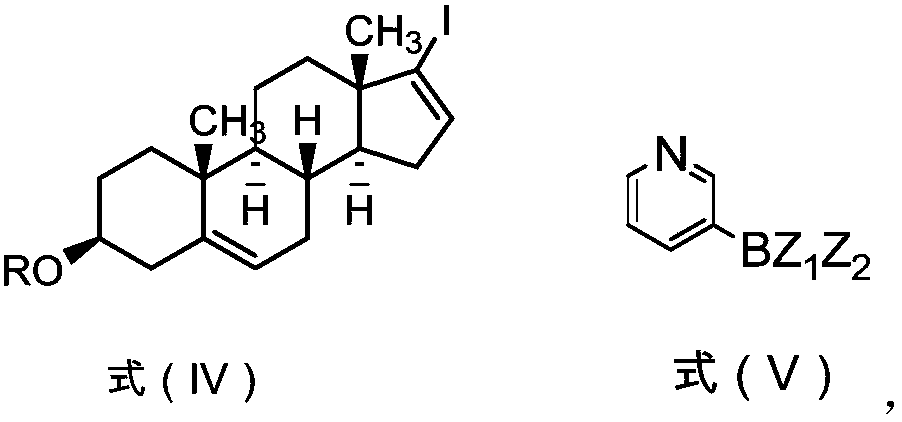
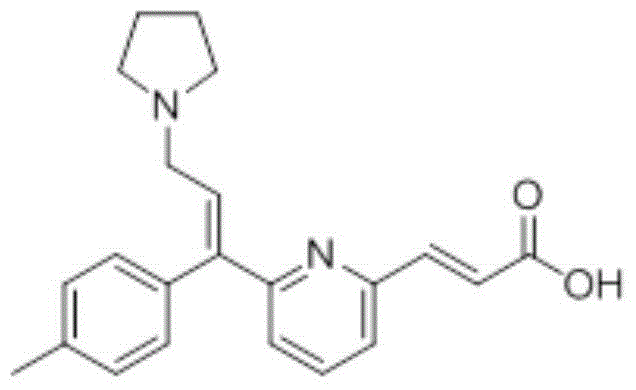
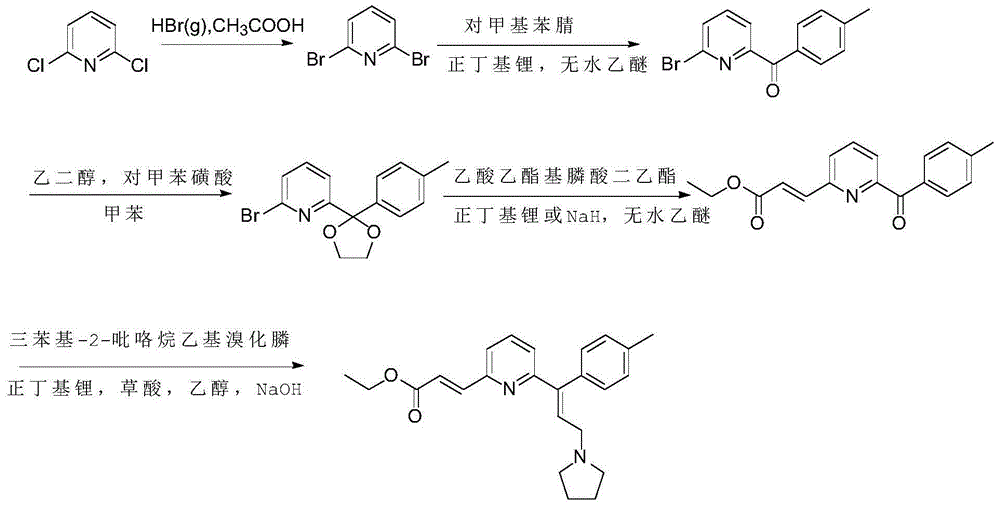
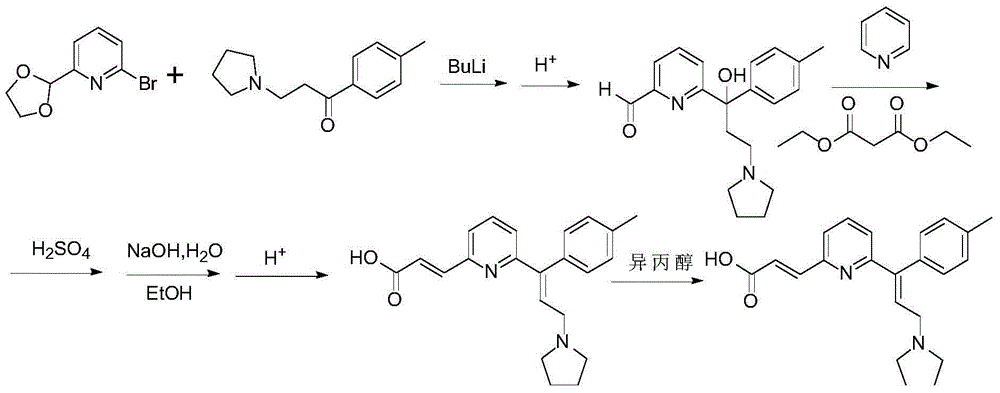
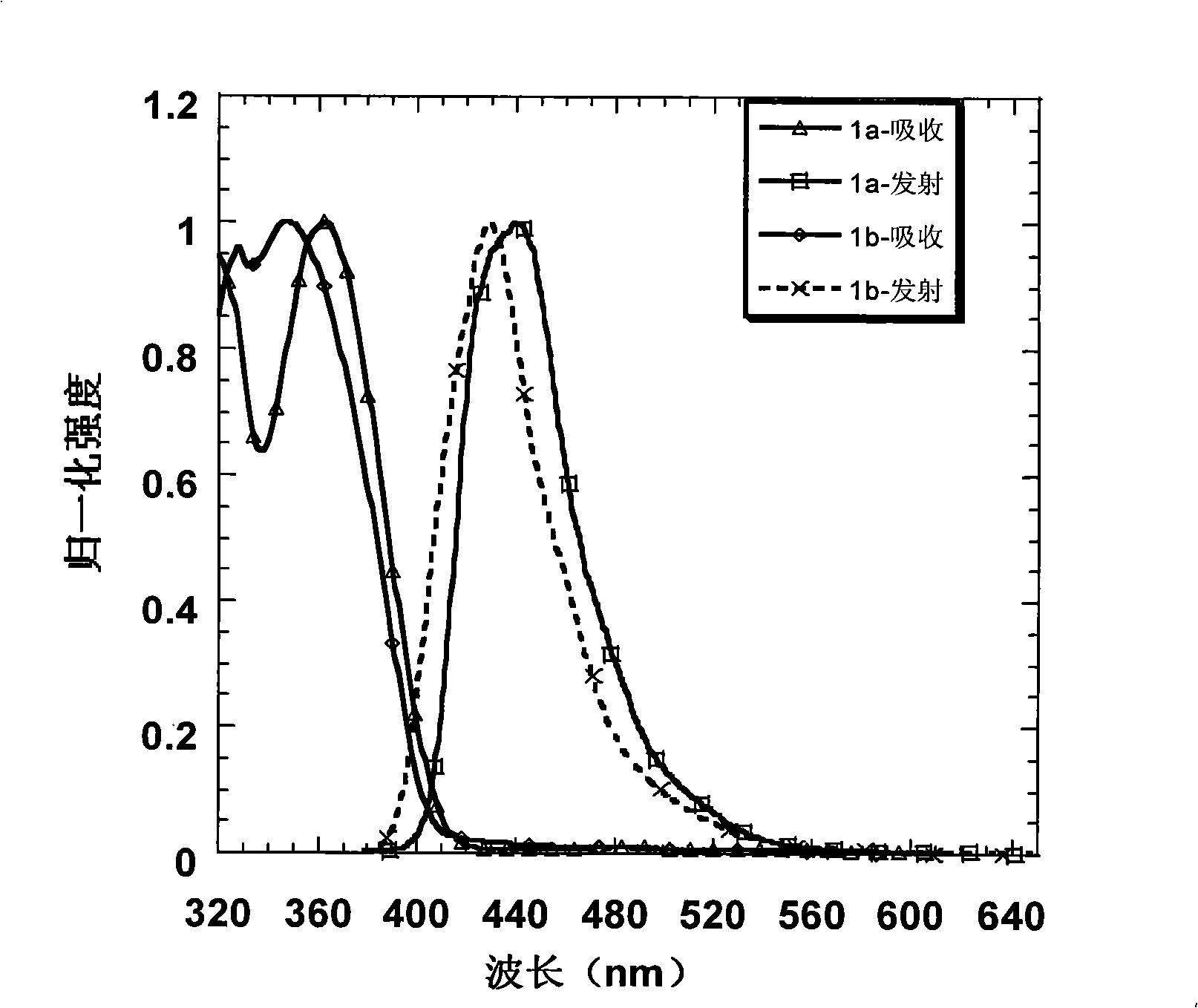
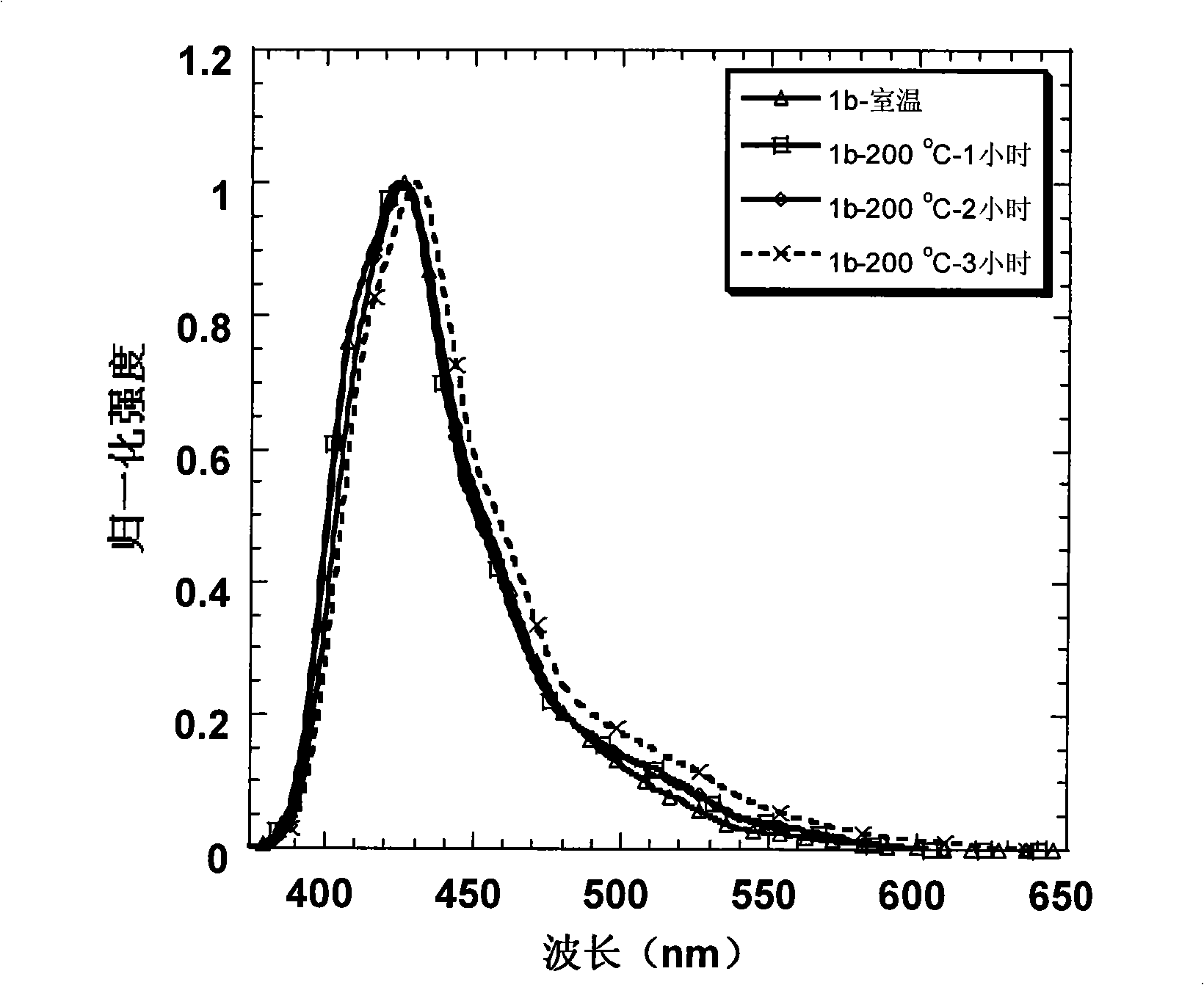
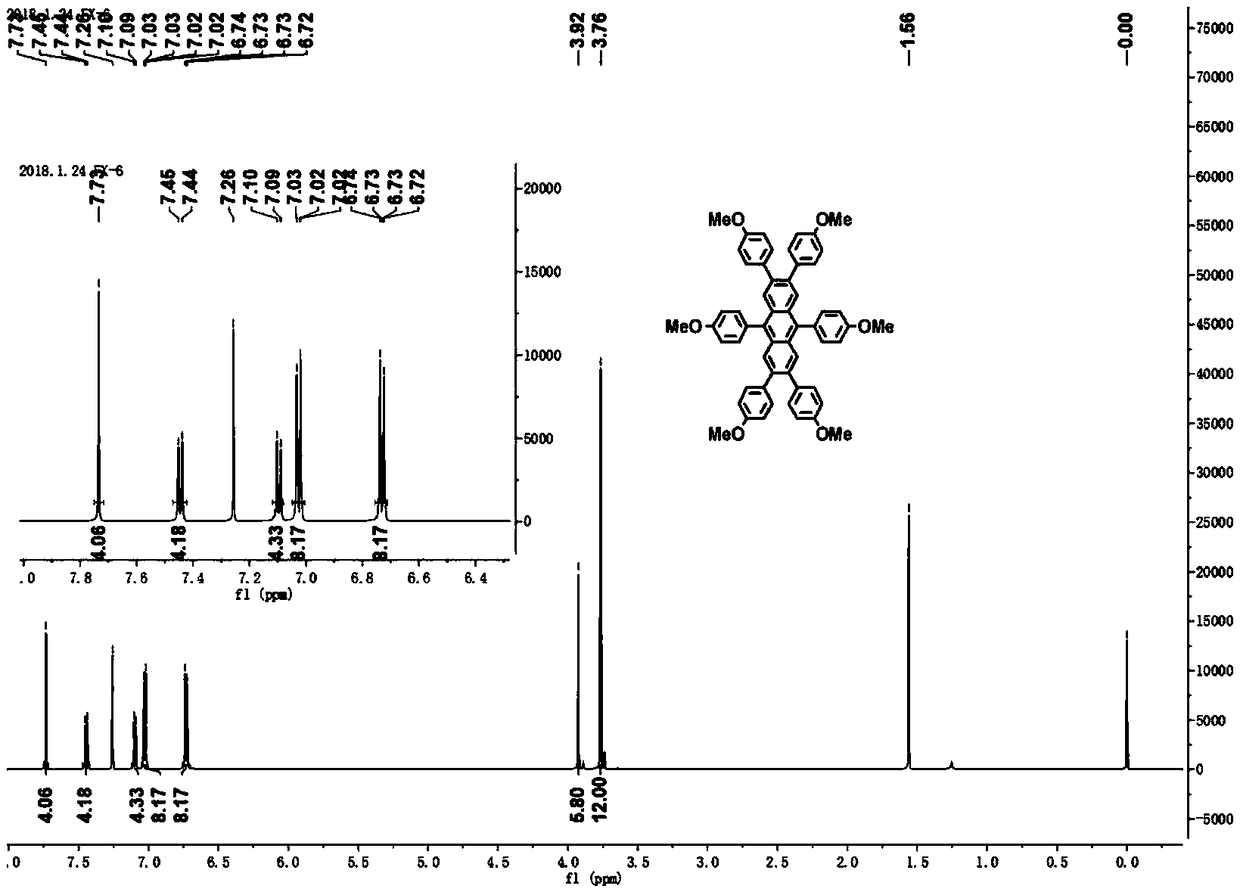
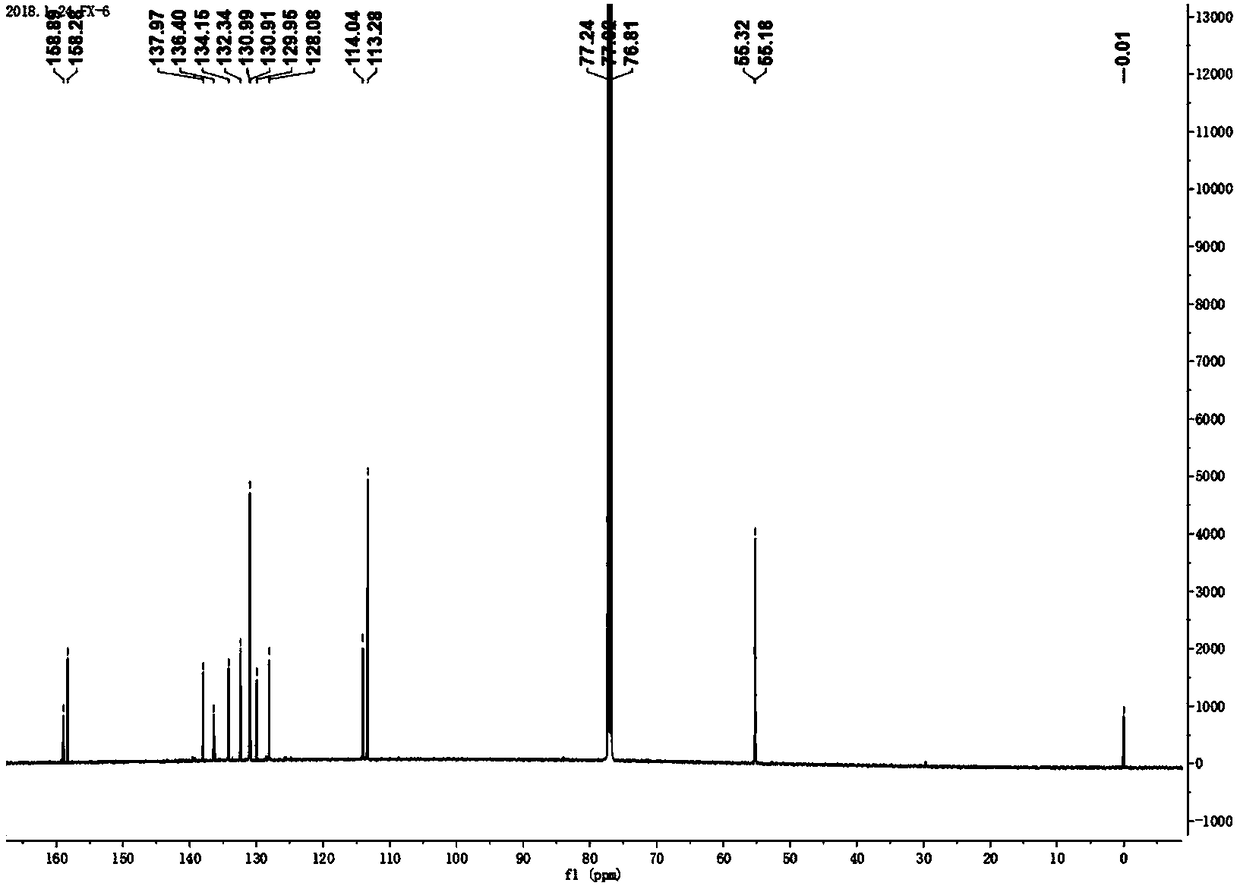
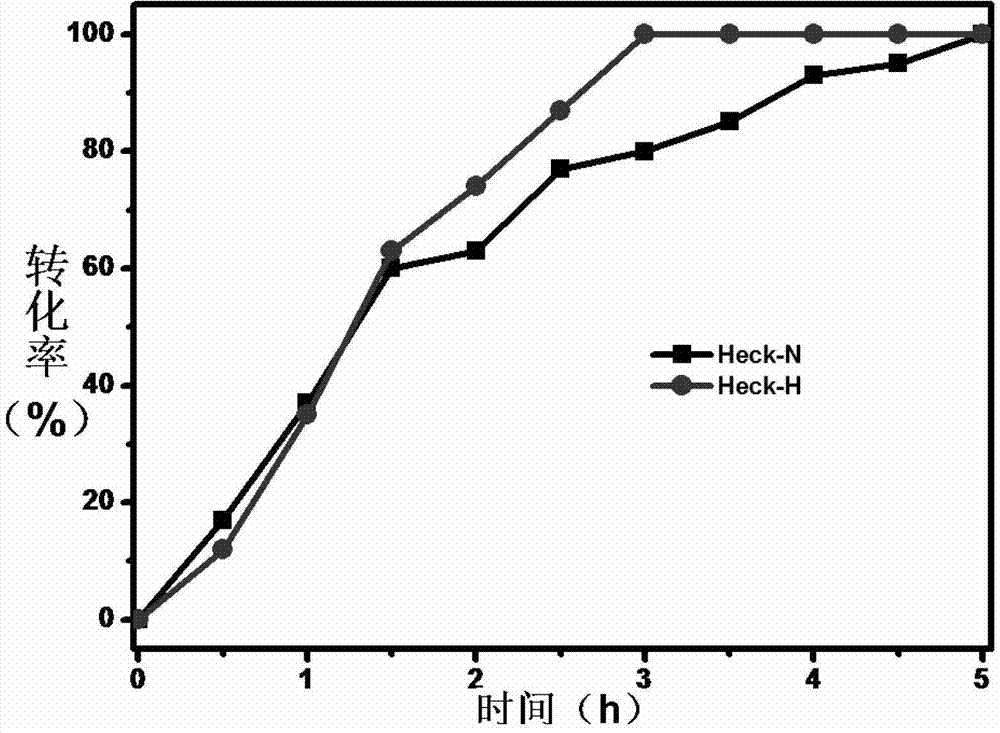
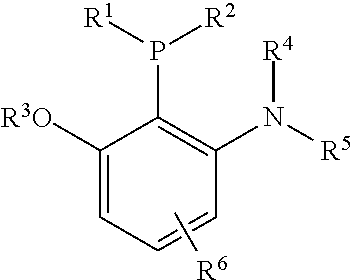
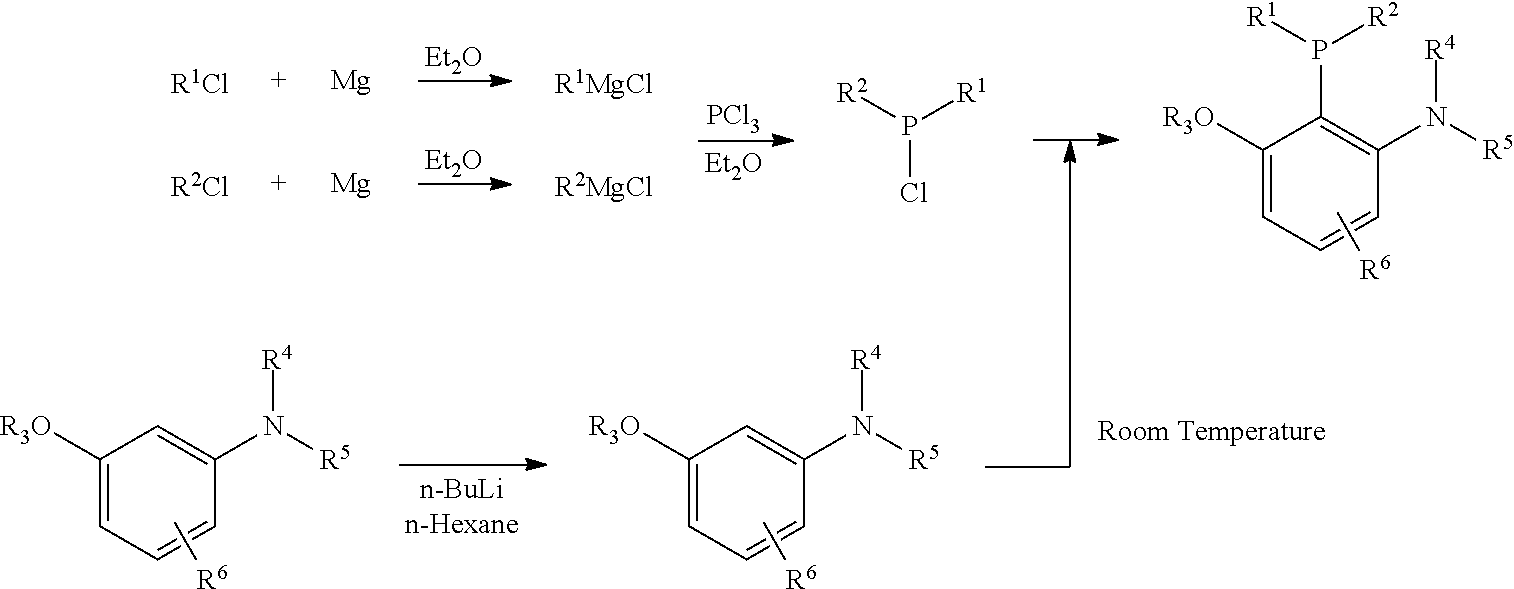
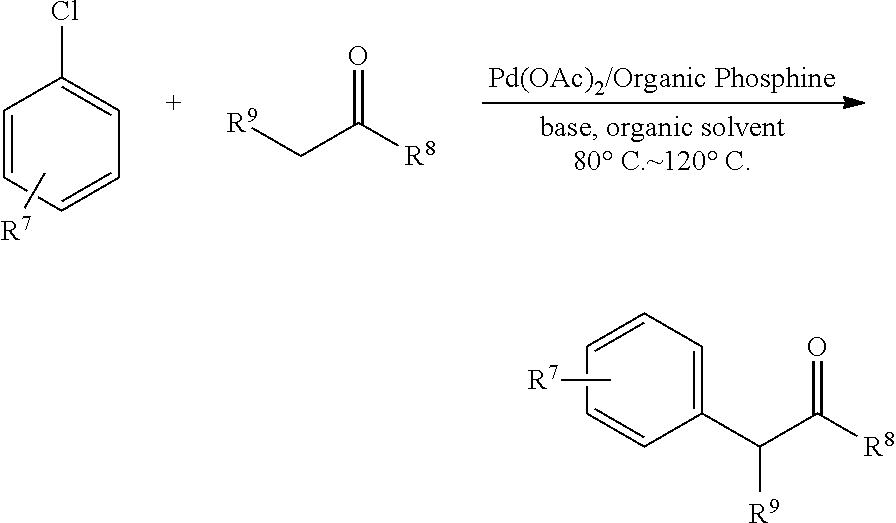
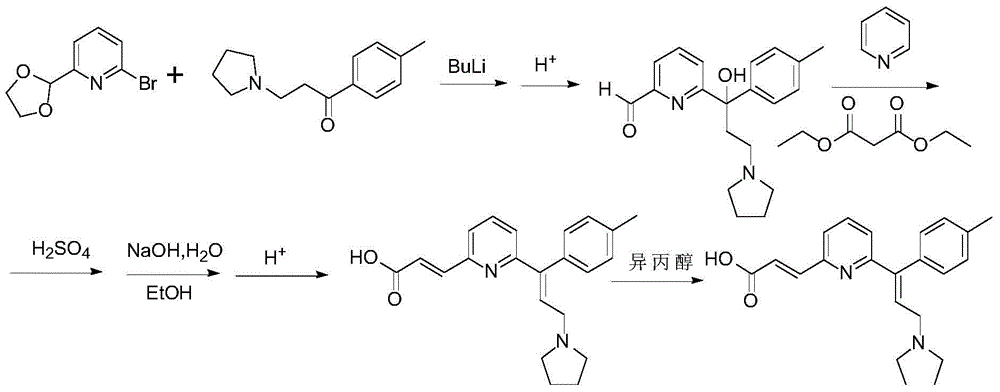
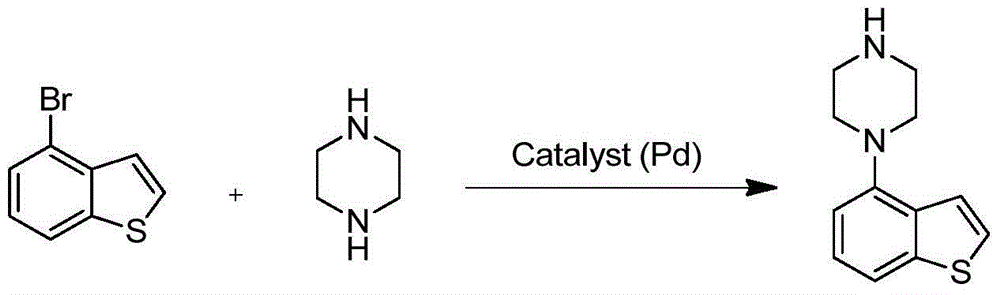
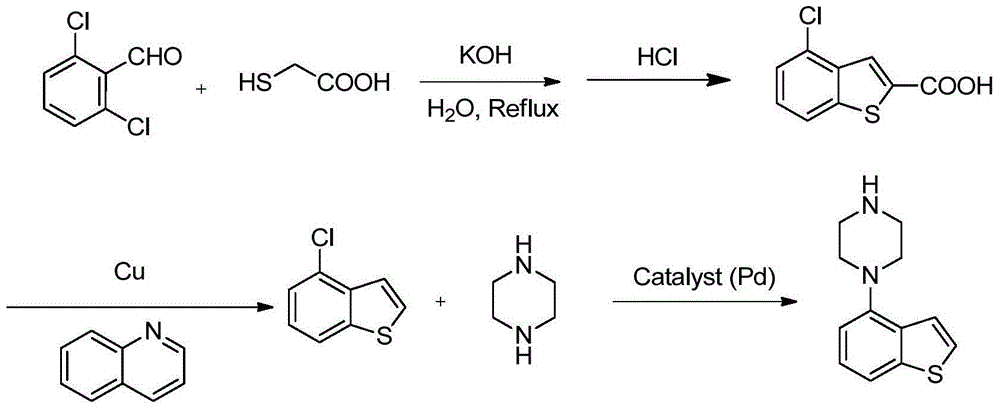
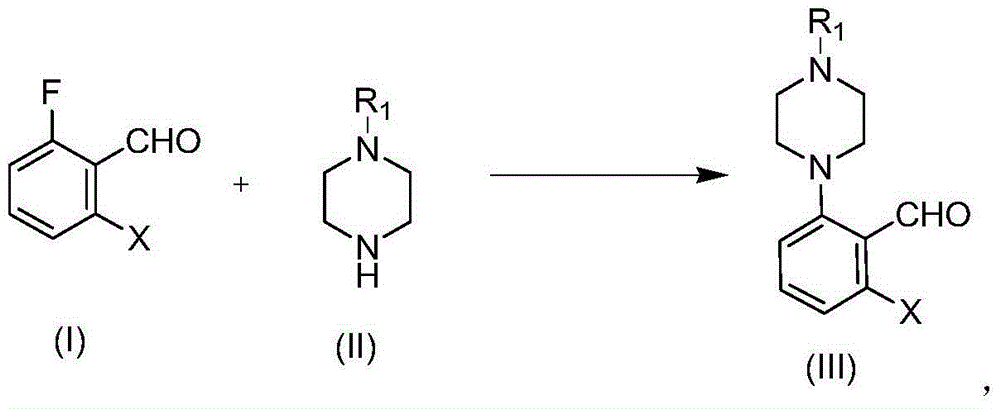
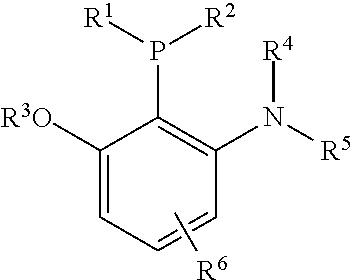
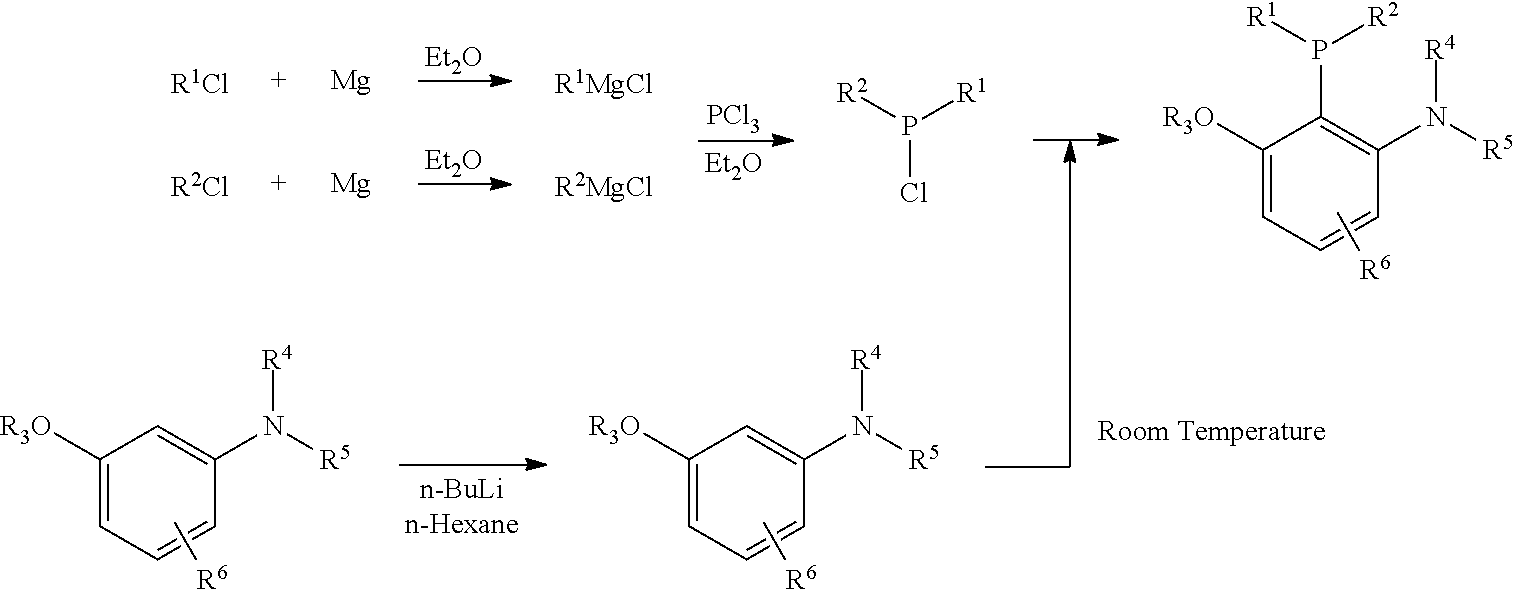
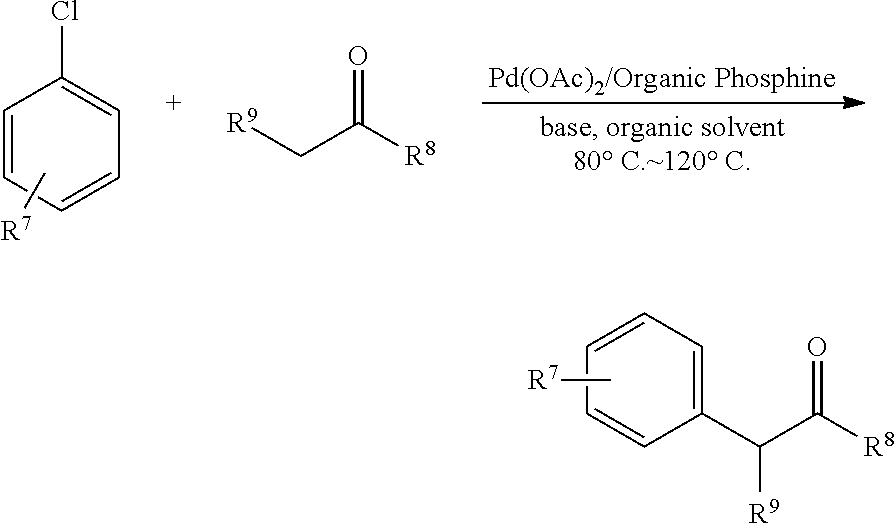
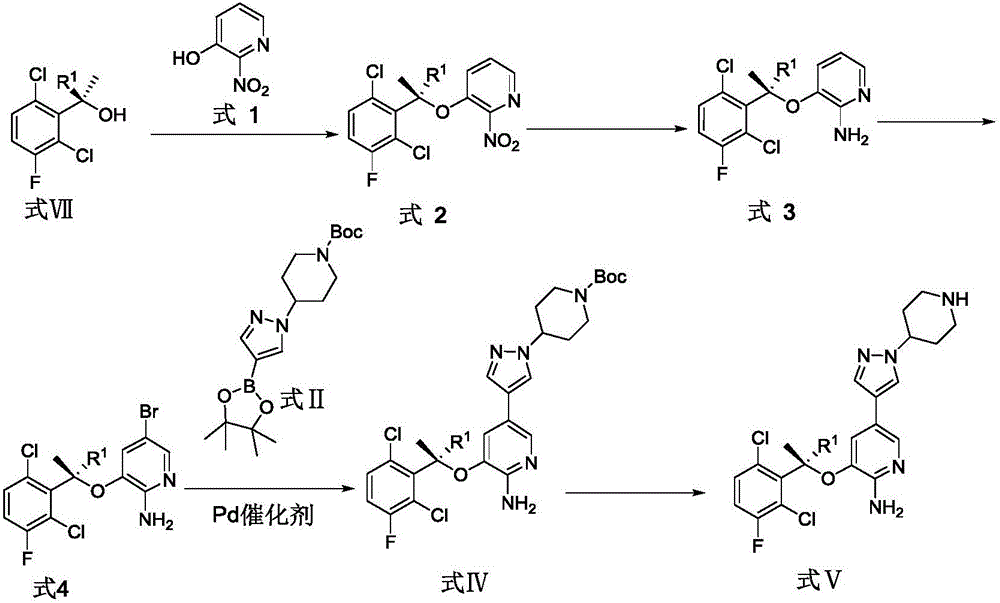
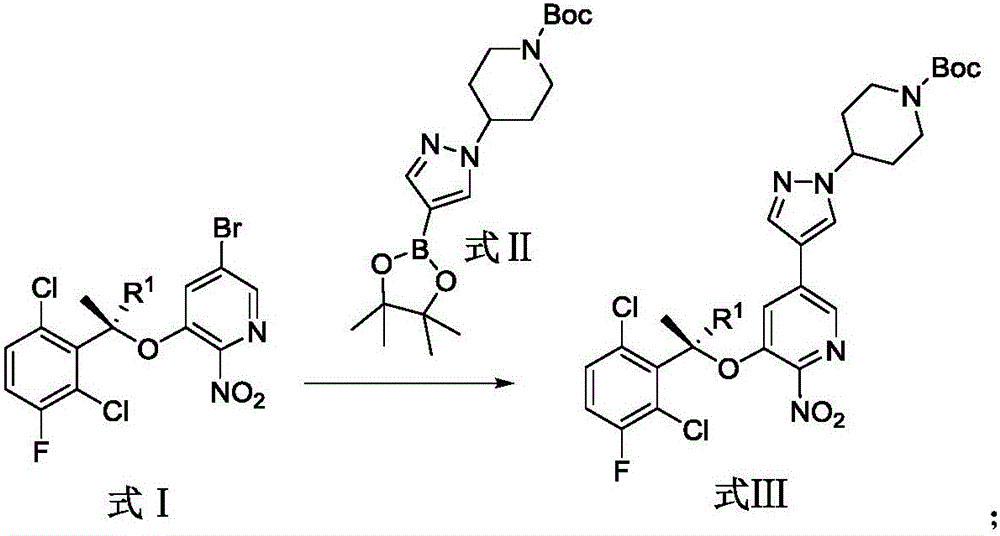
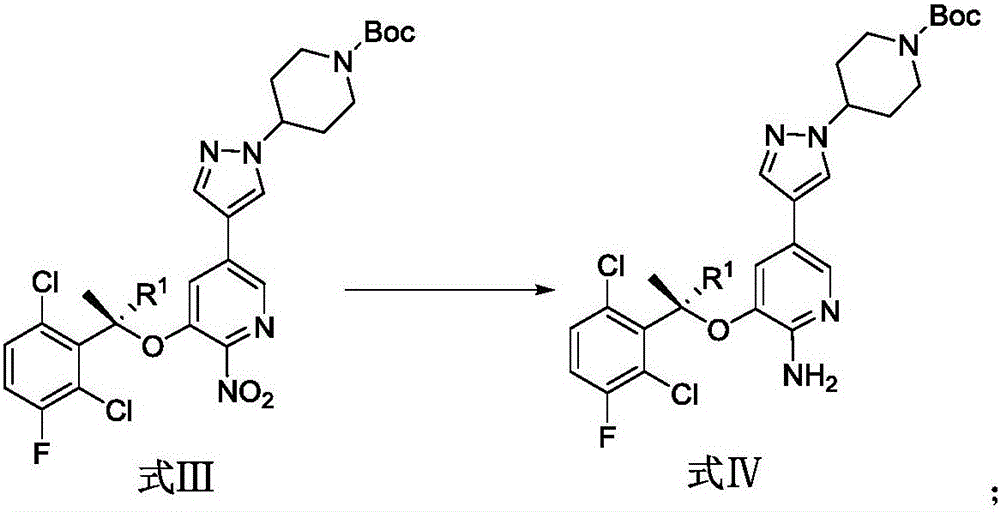
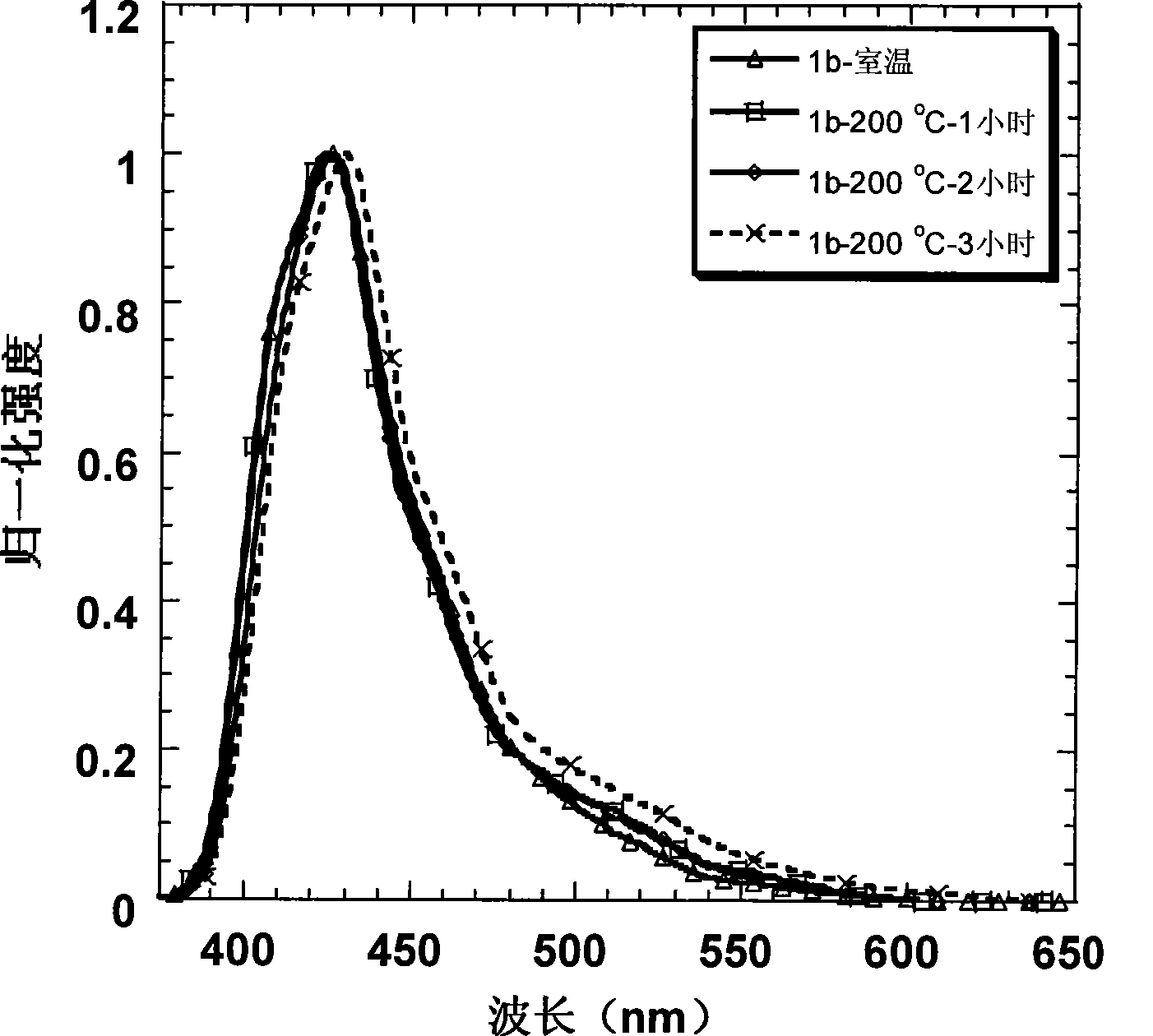
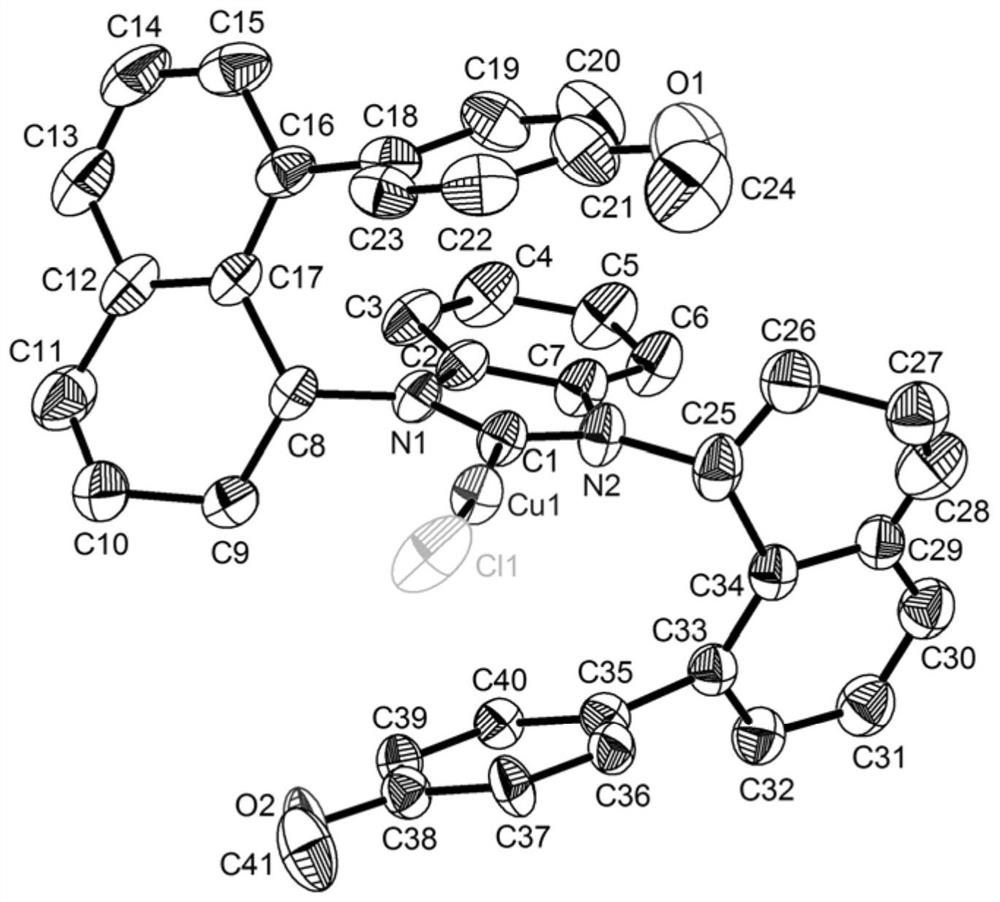
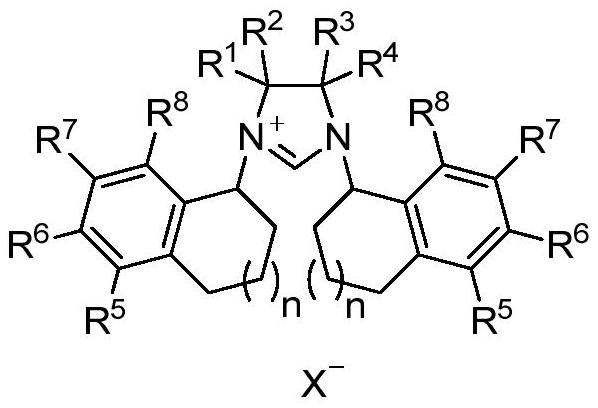
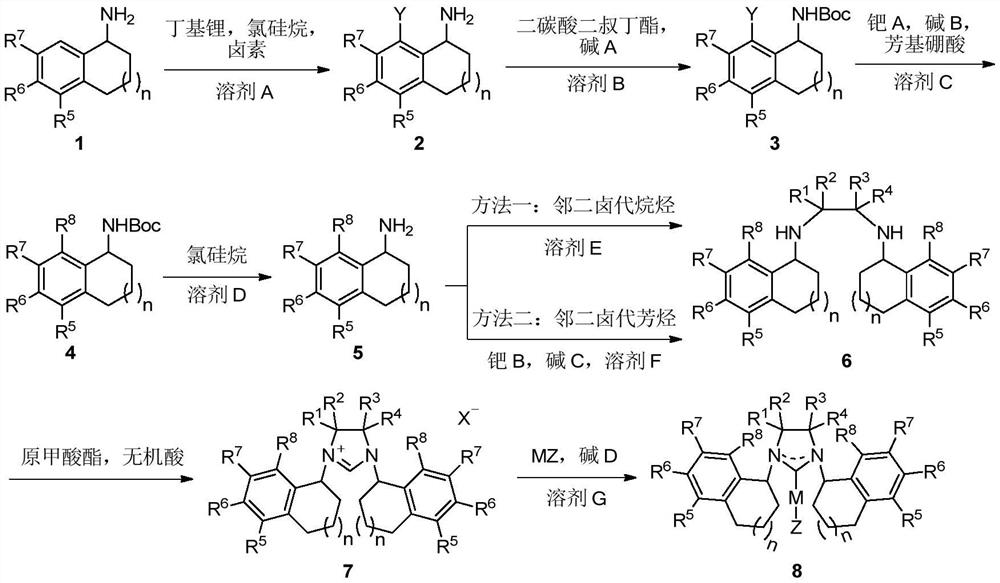
Patents
Literature
32 results about "Palladium-catalyzed coupling reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A cross-coupling reaction in organic chemistry is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon-carbon bond in the product R-R'. Cross-coupling reaction are a subset of coupling reactions. It is often used in arylations.
Soluble electro-green light organic molecule glass material and preparation method and use thereof
InactiveCN101392174AGood holeFacilitates electron injectionOrganic chemistrySolid-state devicesSolubilityCuprous salt
The invention discloses a soluble electroluminescent green light organic molecule glass material, a preparation method and applications thereof. The material comprises two types of a symmetric substituent benzothiadiazole derivative and a dissymmetric substituent benzothiadiazole derivative. The preparation method of the material comprises: carbazol and fluorine or anthracene are taken as raw materials, a bromide that contains Ar1 is obtained through palladium-catalyzed coupling reaction or cuprous salt catalyzed coupling reaction, and corresponding boric acid ester is generated through a next reaction; the boric acid ester is reacted with 4, 7-dibromo benzothiadiazole or a bromide of benzothiadiazole substituted by soluble resin Ar2 and a tiny molecule luminescent material which is symmetric or dissymmetric is obtained. The luminescent material prepared has good solubility in a solvent with high boiling point and weak polarity and can be purified by the solution method; simultaneously, the luminescent material has good thermal stability and morphologic stability, particularly the luminescent material with the dissymmetric structure has advantages in both synthesis and purification, thus having important application prospect in electroluminescence display, illumination and laser.
Owner:SOUTH CHINA UNIV OF TECH
Trifluoreneamine compound, trifluoreneamine polymer luminescent material and preparation methods and application thereof
InactiveCN102093232AEasy to synthesizeEasy to purifyCarboxylic acid nitrile preparationOrganic compound preparationSide chainPhotoluminescence
The invention discloses a trifluoreneamine compound, a trifluoreneamine polymer luminescent material and preparation methods and application thereof. The material takes trifluoreneamine as a framework; the bipolarity and the illuminant color of the luminescent material can be regulated and controlled by changing the chemical structure of a side chain of the trifluoreneamine polymer luminescent material; and the chemical structure of the material is shown in the specifications. The preparation method comprises the following steps of: performing a series of simple reactions on fluorene serving as an initial reaction raw material; and performing a Suzuki coupling reaction under the catalysis of palladium and performing double-boric acid ester alternative copolymerization on fluorene to obtain a target polymer, namely the luminescent material. The material is easy to synthesize, is convenient to purify, has higher solubility, film forming property, and film form stability and high photoluminescence and electrofluorescence efficiencies, and is applied to a polymer electroluminescent diode luminous layer.
Owner:SOUTH CHINA UNIV OF TECH +1
Method for preparing phenylamide compound
InactiveCN102432488AOrganic compound preparationCarboxylic acid amides preparationReaction temperatureSilica gel
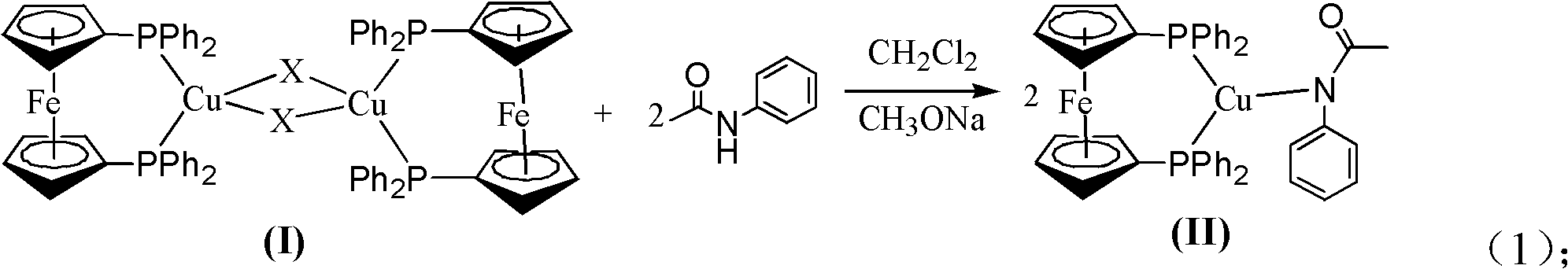
The invention relates to a method for preparing a phenylamide compound, which is characterized by comprising the following steps of: (1) adding aryl halide and amide or an amide derivative into a reaction vessel, adding cuprous halide and 1,1'-bis(diphenylphosphino)ferrocene serving as catalysts, adding alkali and an organic solvent, and reacting at the temperature of between 50 and 150 DEG C for 2 to 24 hours; and (2) filtering reaction liquid obtained in the step (1), purifying by using a silica gel column of 200 to 300 meshes, pre-leaching the silica gel column by using 20 to 50mL of petroleum ether, eluting by using a leacheate at a flow rate of 1 to 2mL / min for 3 to 6 hours, removing the solvent, and thus obtaining the phenylamide compound. By the method, the defects of high temperature and long reaction time of copper-catalyzed coupling reaction and high cost and environmental pollution of palladium-catalyzed coupling reaction are overcome.
Owner:JIANGNAN UNIV
Polymer semiconductor containing bipyridine diazole derivative receptor and preparation method and application thereof
ActiveCN107573489AUniversalHigh synthetic yieldSolid-state devicesSemiconductor/solid-state device manufacturingPolymer scienceTri-n-butyltin
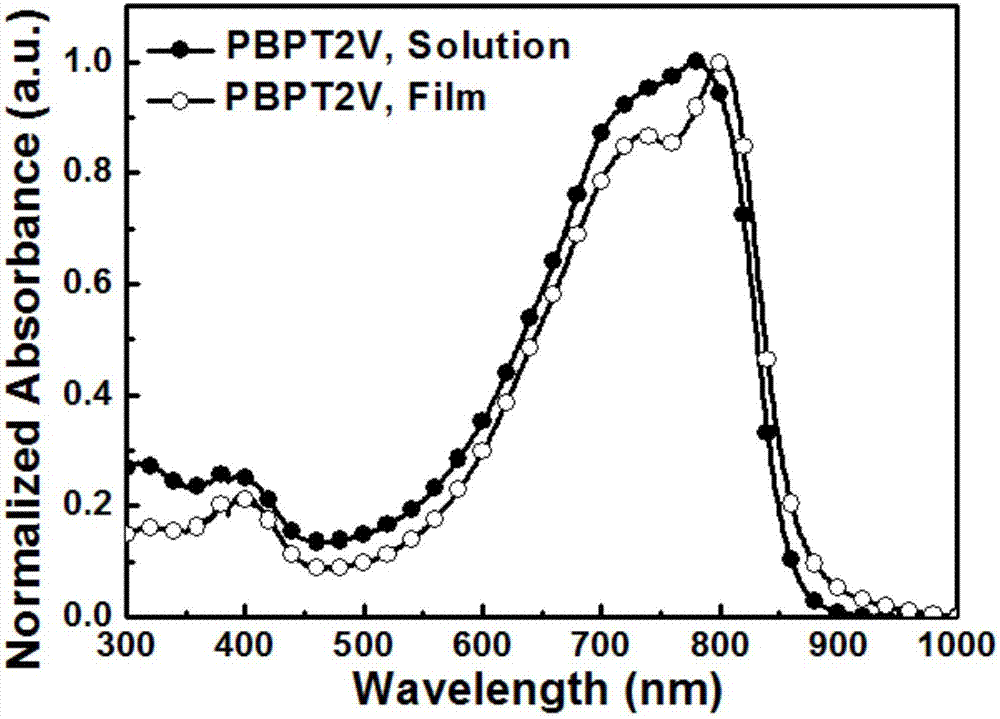
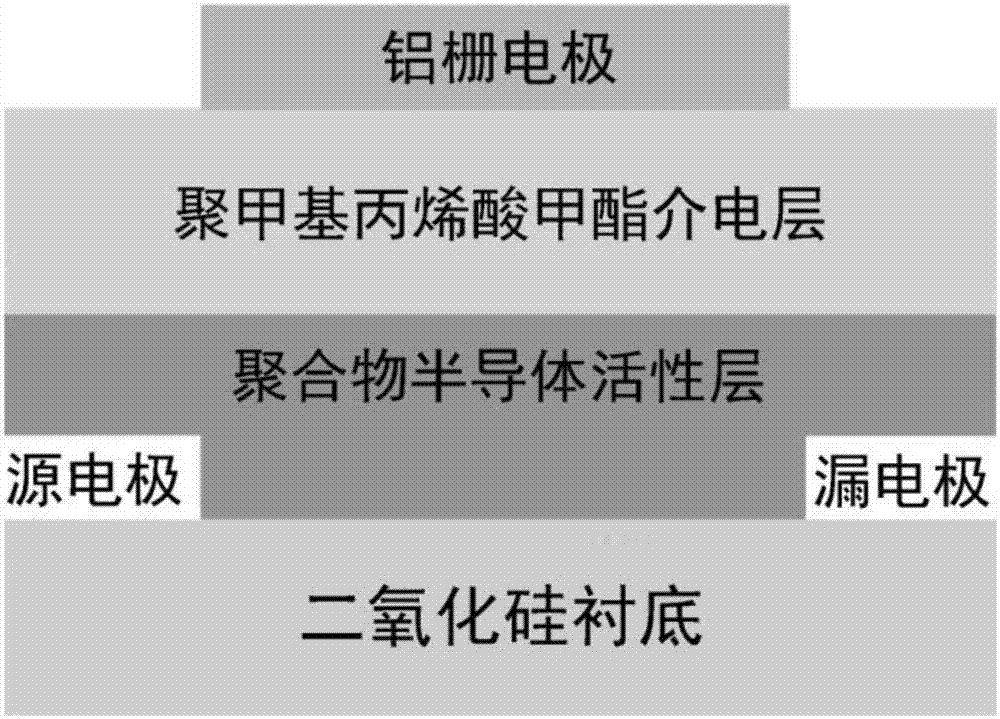
The invention belongs to the field of polymer semiconductor materials, and discloses a polymer semiconductor containing a bipyridine diazole derivative receptor and a preparation method and application thereof. A structure of the polymer semiconductor is shown in a formula (I), and the preparation method comprises the following steps: (1) reacting 2-tributyltin-4-alkylthiophene with a dibromobenzimidazole derivative to prepare an intermediate a; (2) reacting the compound a with a bis-butyltin b to obtain a compound c; (3) performing a reaction on the compound c and N-bromosuccinimide to obtainM1; and (4) performing a palladium-catalyzed coupling reaction on the M1 and (E)-bis1,2-(tri-n-butyltin)ethylene to obtain the final product. According to the invention, the synthetic route is simpleand high-efficiency, the raw materials are easy to obtain, the costs are low, the universality is high and the repeatability is good, and a field effect transistor prepared by using the polymer semiconductor as an active layer obtains outstanding bipolar transmission characteristics, and has wide market prospects.
Owner:XIANGTAN UNIV
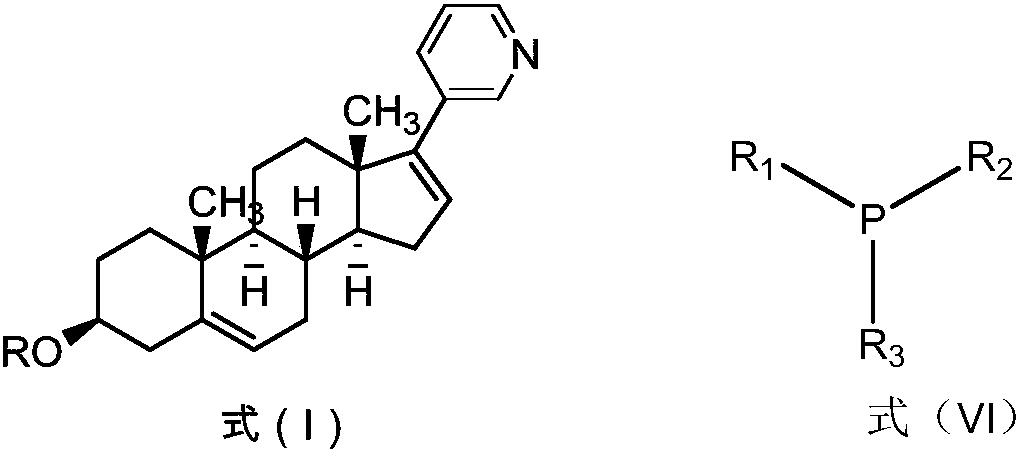
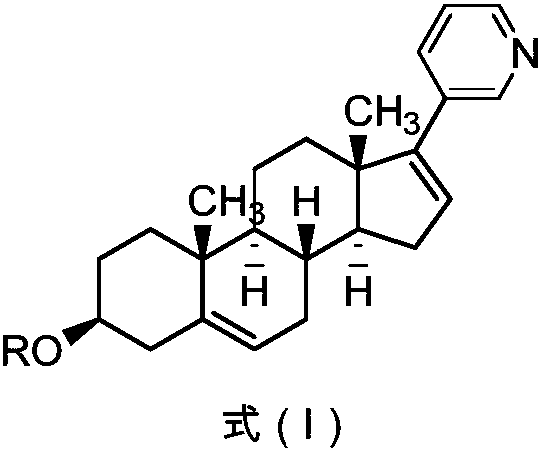
Abiraterone acetate preparation method
PendingCN107840866AOrganic compound preparationSteroidsPalladium-catalyzed coupling reactionsPhosphine
The invention discloses an abiraterone acetate preparation method, and particularly provides an abiraterone acetate preparation process. In the prior art, the preparation process comprises a palladiumcatalyzed coupling reaction, the palladium catalyzed reaction can cause the pollution on the reaction product, and palladium is difficultly separated from the target product. According to the presentinvention, palladium is removed with the substituted phosphine-containing compound so as to effectively control the palladium residue.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Preparation method of acrivastine
The invention belongs to the field of organic chemical synthesis and specifically relates to a preparation method of acrivastine. The preparation method comprises the following steps: by taking p-methyl benzoyl hydrazone and alpha-halogenated pyridine as raw materials, carrying out palladium-catalyzed coupling reaction to obtain an intermediate for synthesizing acrivastine; and further preparing acrivastine. By carrying out non-palladium borate-catalyzed coupling reaction, the preparation method is mild in reaction condition; dangerous butyl lithium is not used, so that the risk in plant production is reduced; the reaction is short in time required and high in efficiency; and the raw materials are cheap and easily available and the cost is lowered. The preparation method provided by the invention provides a novel safe, economic and efficient preparation path for preparation of acrivastine.
Owner:CHONGQING HUAPONT PHARMA
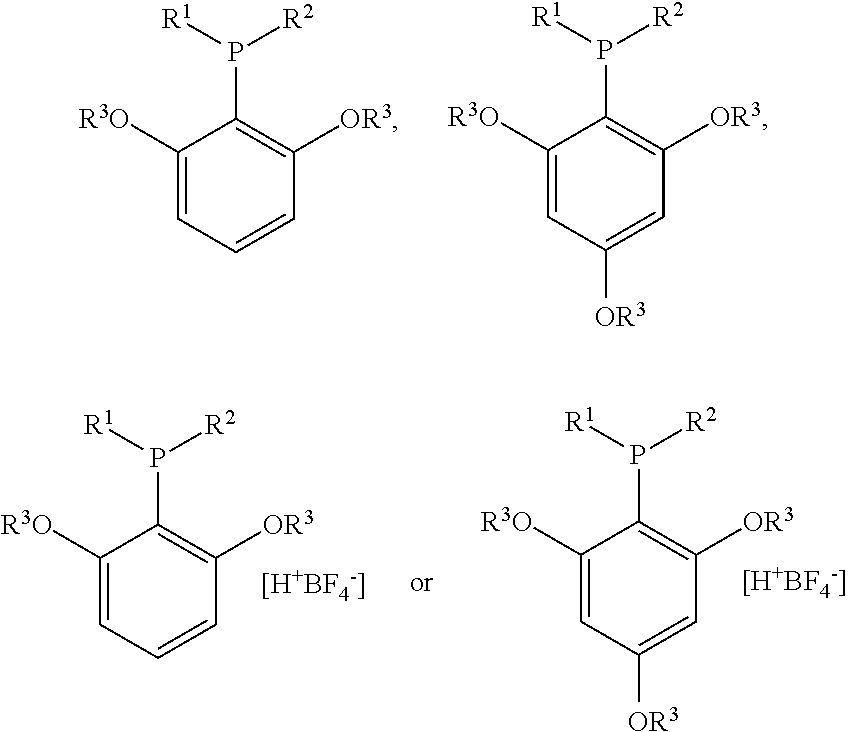
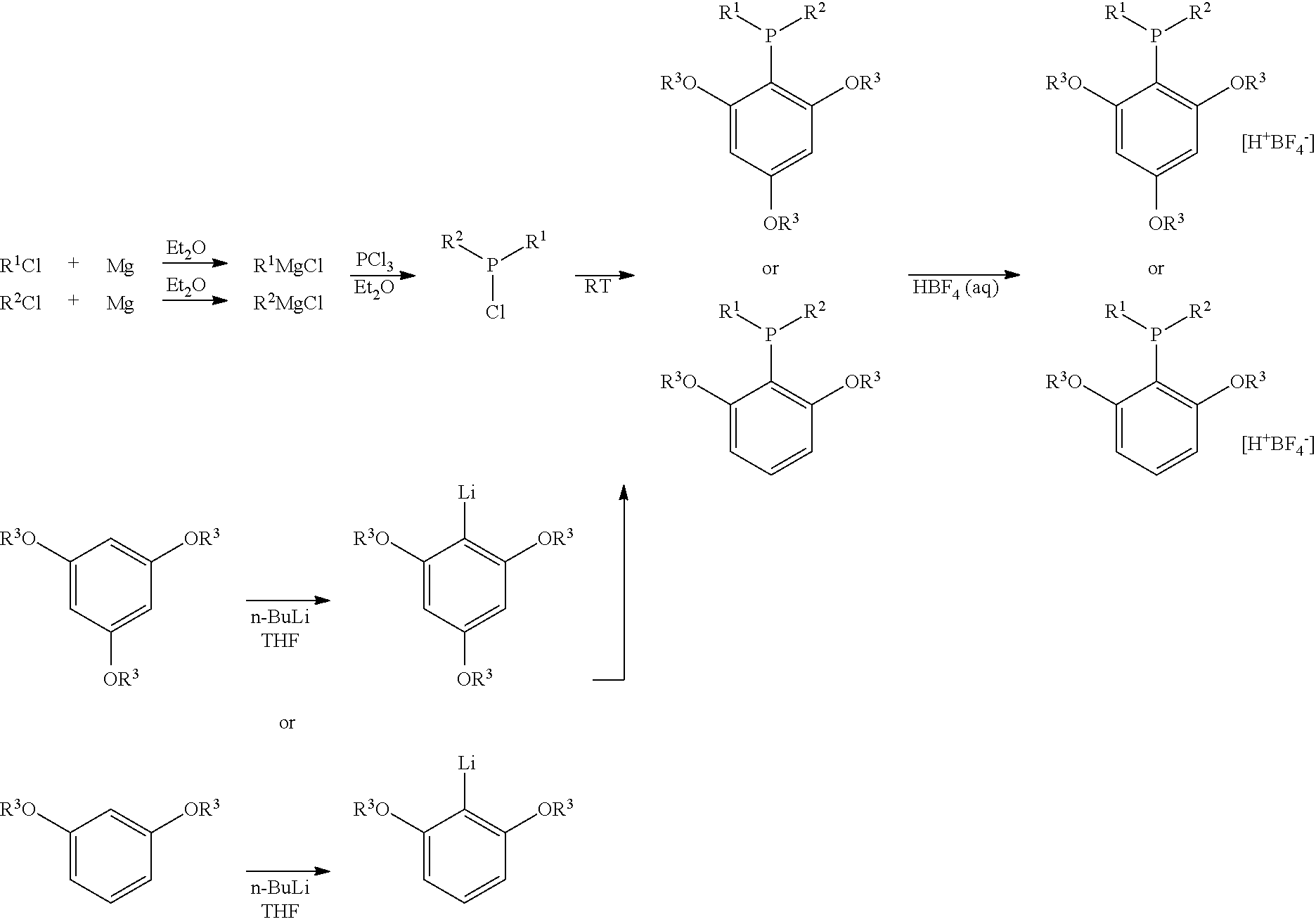
Structure and method for synthesizing and using dialkyl(2,4,6- or 2,6-alkoxyphenyl)phosphine and its tetrafluoroborate
ActiveUS20120197030A1Easy to operateEfficient activationOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsTetrafluoroboratePalladium catalyst
The current invention relates to the structure, synthesis of dialkyl(2,4,6- or 2,6-alkoxyphenyl)phosphine or its tetrafluoroborate, as well as its applications in the palladium catalyzed carbon-chlorine bond activation for Suzuki coupling reactions and carbon-nitrogen bond formation reactions. The dialkyl(2,4,6- or 2,6-alkoxyphenyl)phosphine or its tetrafluoroborate could coordinate with the palladium catalyst to activate the inert carbon-chlorine bond highly selectively and catalyze Suzuki coupling reaction with arylboronic acid or carbon-nitrogen bond formation reaction with organic amines. The current invention uses only one step to synthesize dialkyl(2,4,6- or 2,6-alkoxyphenyl)phosphine and its tetrafluoroborate is stable in the air. Compared with known synthetic routes of ligands used in activating carbon-chlorine bonds, the method of current invention is short, easy to operate. Moreover, with this type of ligands, the Suzuki coupling products of optically active chlorolactones and arylboronic acids would maintain their configuration and optical purity.
Owner:ZHEJIANG UNIV
Organic electroluminescence ethereal blue optical material, preparation method and application thereof
InactiveCN101270036AExtended conjugate lengthImprove stabilityOrganic chemistryOrganic compound preparationFluorescenceLewis acid catalysis
The present invention provides organic small pure blue materials with high stability. The pure blue materials have a structure as shown in formula 1; wherein, R1 stands for Pi-conjugated group; R2 stands for hydrogen, alkyl and alkoxy; and R3 stands for hydrogen, alkyl and alkoxy. The acid-catalyzed Friedel-Crafts ring-closure reaction in the molecules and the high-efficiency palladium-catalyzed coupling reaction solve the problem of molecular synthesis. The compounds have a structure with a spiral ring; the molecules consist of two mutually vertical structural units; thus the larger size and the branching structure effectively prevent the aggregation of the molecules; and the high-purity blue light can be prepared. The spiral-ring structure with sp<3> hybridized central carbon atoms high relatively high stability; thus the compounds have excellent stability. The compounds can be used as electroluminescent diodes on the light-emitting layer to send pure blue fluorescence, and have high electric and thermal stability.
Owner:PEKING UNIV
Novel anthracene luminescent material, preparation method, and applications thereof
ActiveCN108689986AHigh luminous intensityHigh color purityOrganic compound preparationCarbonyl compound preparationQuantum yieldComputational chemistry
The invention belongs to the field of organic semiconductor materials, and discloses a novel anthracene luminescent material, a preparation method and applications thereof; the preparation method includes the steps of: in an inert gas atmosphere, performing a bromination reaction to anthracene, 9-bromoanthracene or 9,10-dibromoanthracene as raw material with addition of a bromination agent under catalysis by metal or Lewis acid; then performing palladium-catalyzed coupling reaction to introduce substituent groups on the 2nd, 3rd, 6th, 7th, 9th and 10th or 2nd, 3rd, 6th, 7th and 9th loci of theanthracene. The luminescent material has a symmetrical functionalized molecular structure of anthracene, so that luminescent intensity, color purity and stability are improved. For the first time, through catalysis, the substituent groups are introduced onto the 2nd, 3rd, 6th, 7th, 9th and 10th or 2nd, 3rd, 6th, 7th and 9th loci of the anthracene, so that molecular planarity is damaged and [pi]-[pi] stacking is inhibited, thus improving the luminescent efficiency and quantum yield performance of the material are enhanced, controlling the luminescent range of the material, inhibiting crystallization, improving film-forming property and improving charge transmission performance.
Owner:GUANGDONG UNIV OF TECH
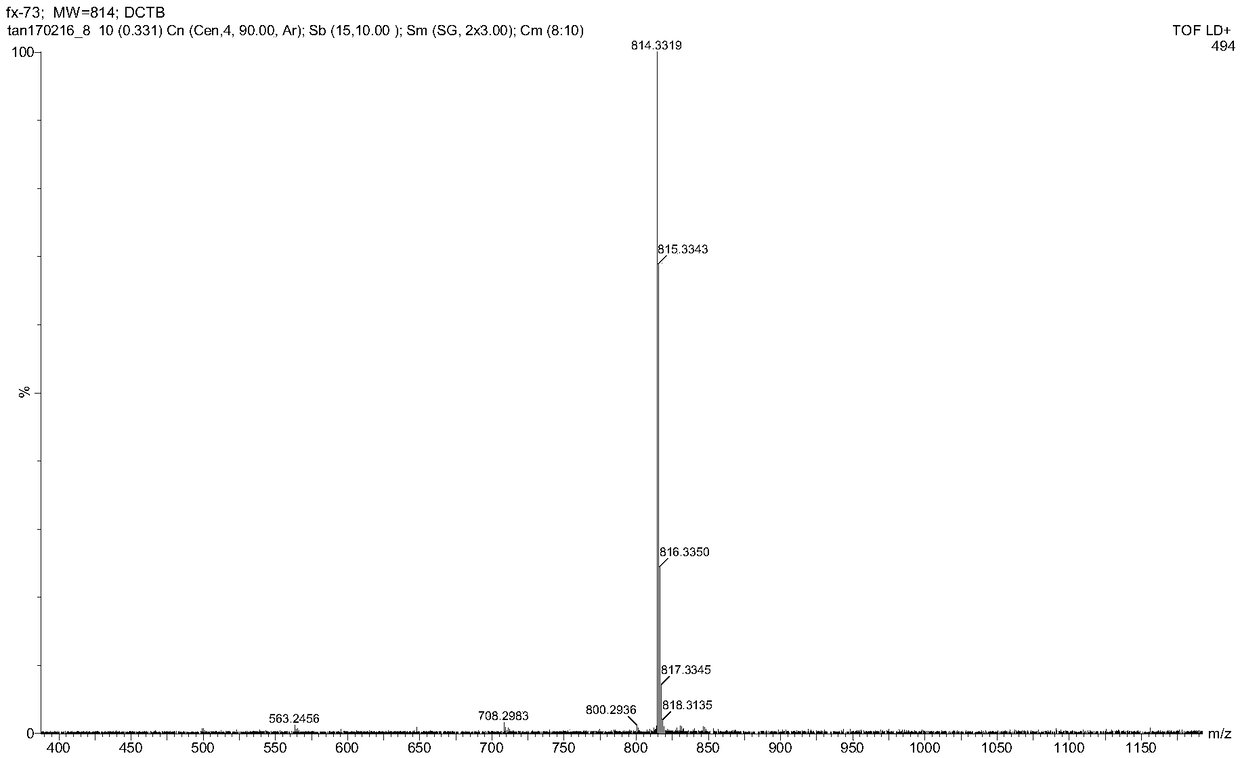
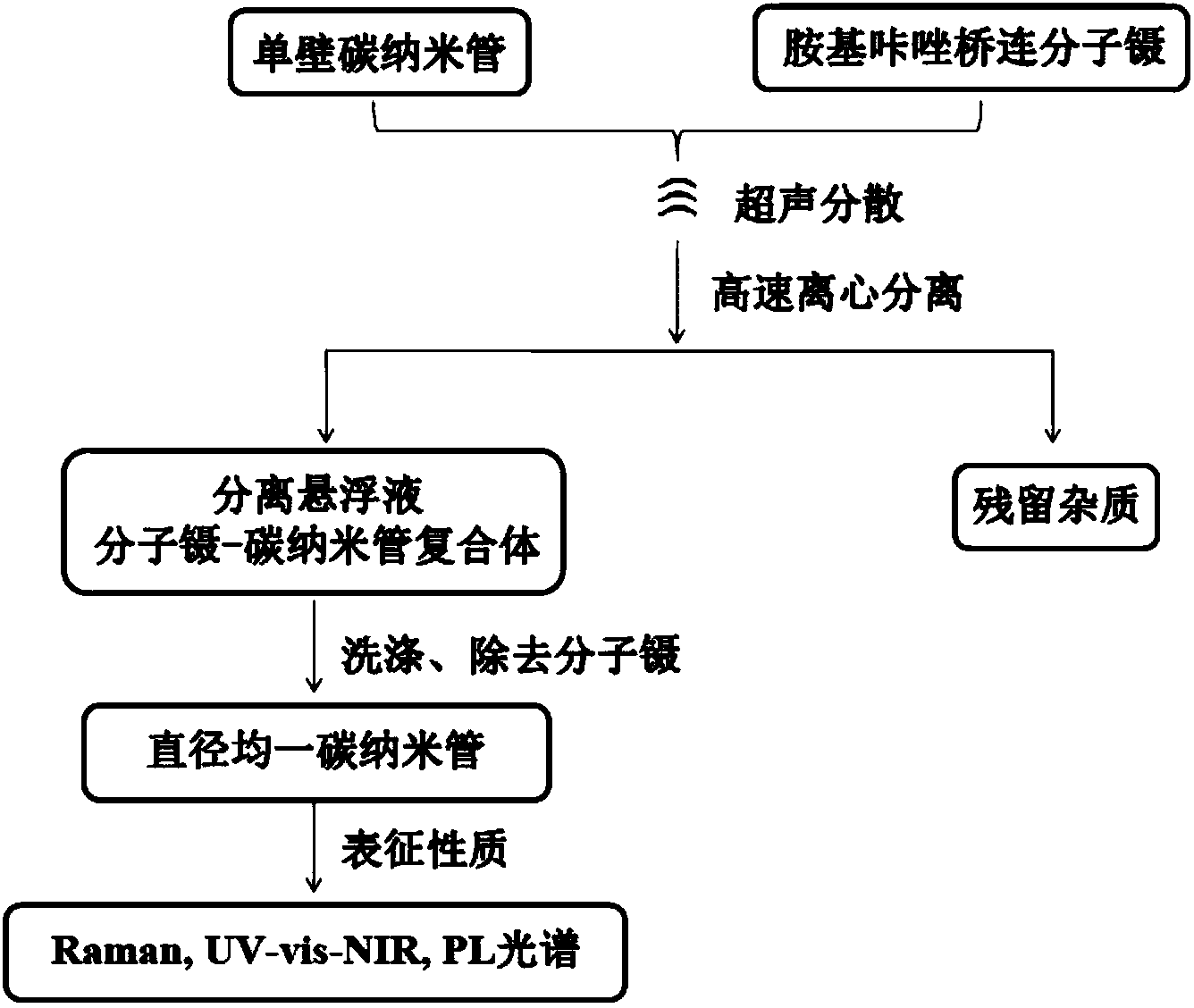
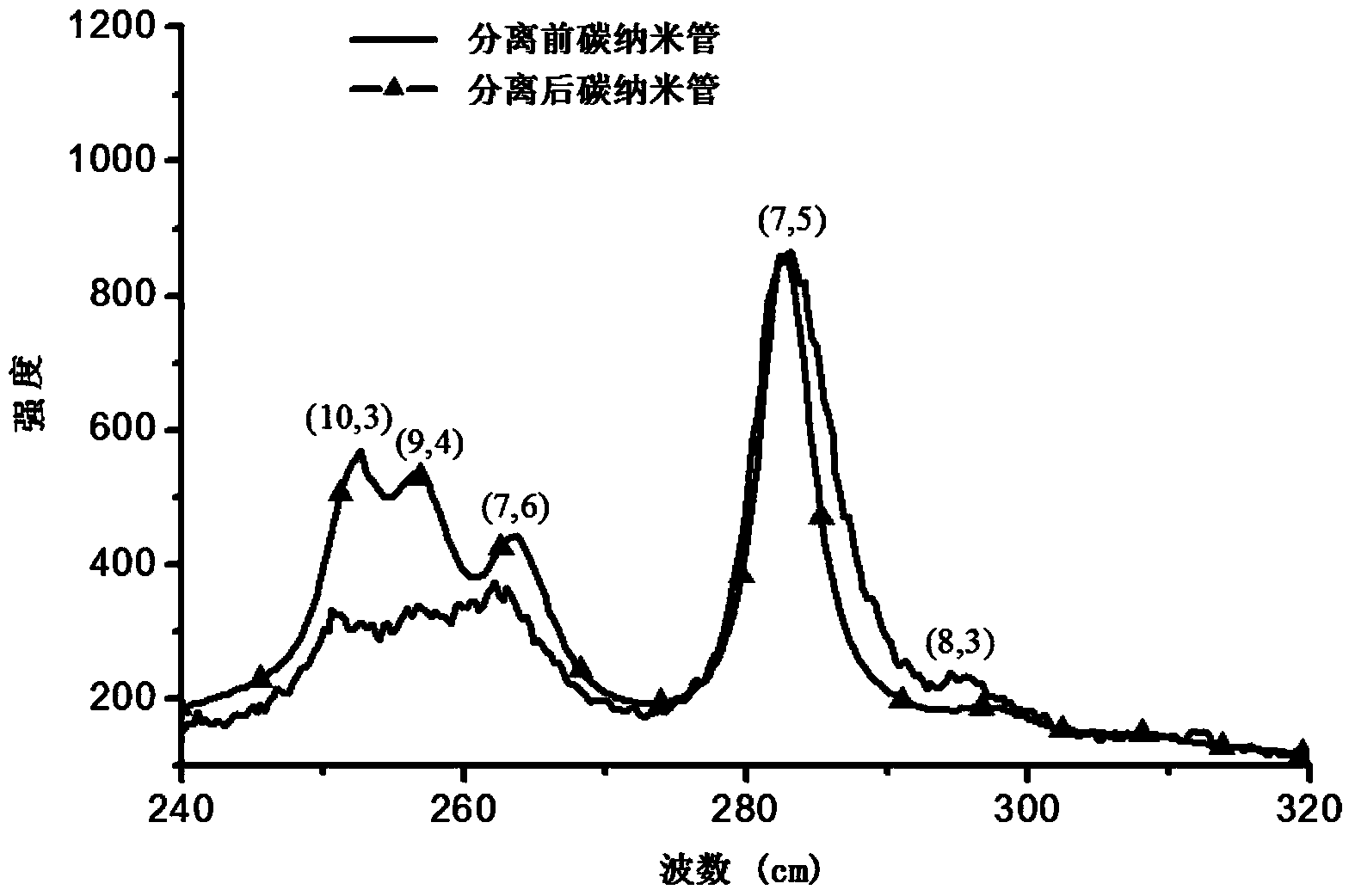
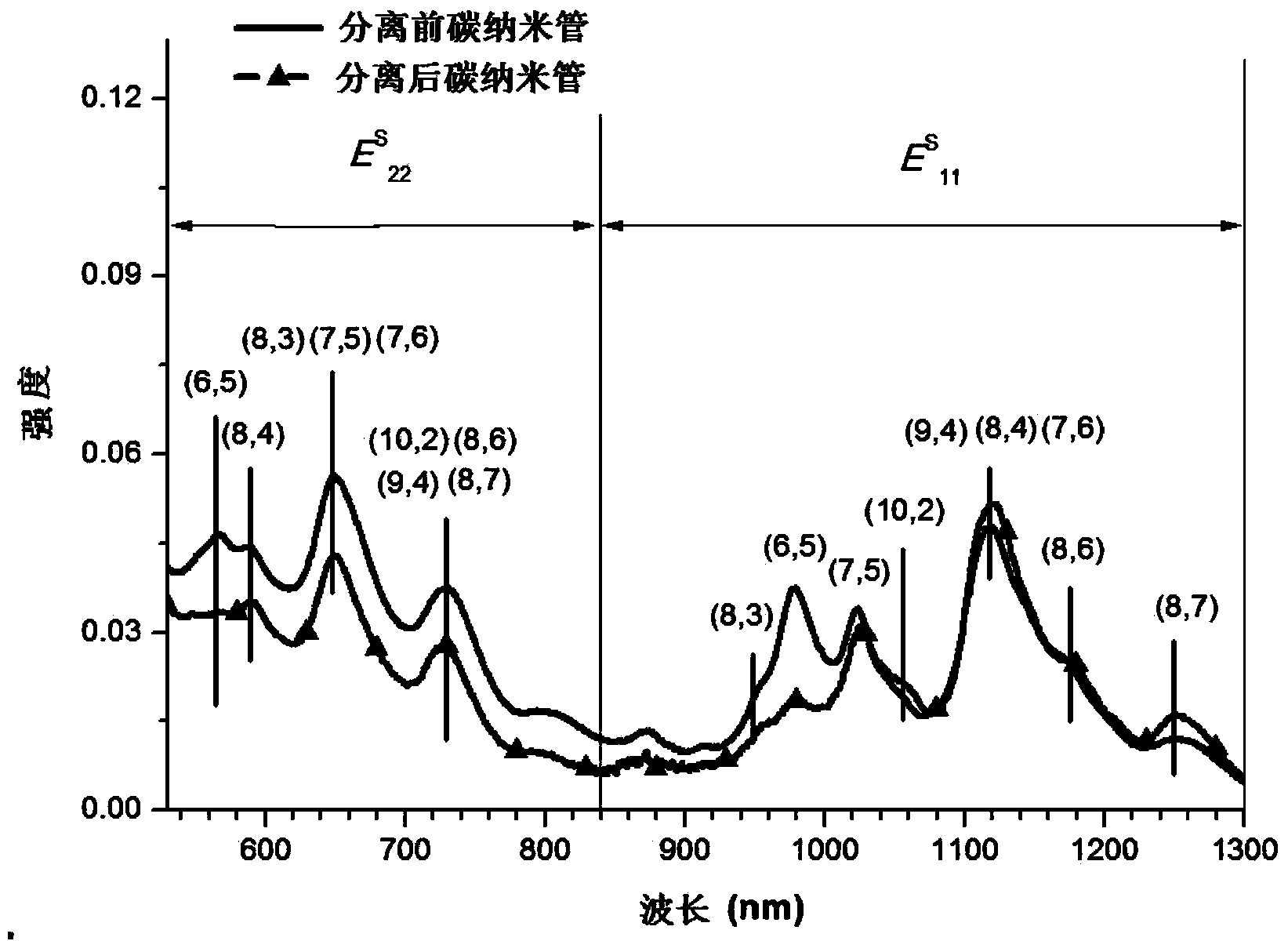
Aminocarbazole bridged molecular tweezer, and preparation method and applications thereof
InactiveCN104341335AEasy to synthesizeLow costMaterial nanotechnologyOrganic chemistryChemical structureSolubility
The invention relates to an aminocarbazole bridged molecular tweezer, and a preparation method thereof. The aminocarbazole bridged molecular tweezer has the chemical structural formula shown in the following general formula which is shown in the specification, wherein Ar is condensed ring pyrenyl; n is equal to 1-15; R1 and R2 are alkyl chains with C1-C6. The preparation method comprises the following steps: 1) by taking 3,6-dibromocarbazole as a reaction raw material, reacting with excessive amine compounds under basic condition to obtain different aminocarbazole dibromides; and 2) by taking the aminocarbazole dibromides as raw materials, introducing rigid condensed ring pyrenyl units through palladium-catalyzed coupling reaction to obtain different target products. The aminocarbazole bridged molecular tweezer and the preparation method and applications thereof have the following advantages and beneficial effects that the aminocarbazole bridged molecular tweezer is simple to synthesize, low in cost, good in separation effect, and beneficial in large-scale purification of carbon nanotubes, has better alcohol solubility, is capable of separating the carbon nanotubes in an environment-friendly type solvent medium of ethanol, propanol and the like, and has the characteristics of being environment-friendly, strong in practicability and the like.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Racemic and chiral 3-(2, 3-butadienyl)oxoindolone compound, and preparation method and application thereof
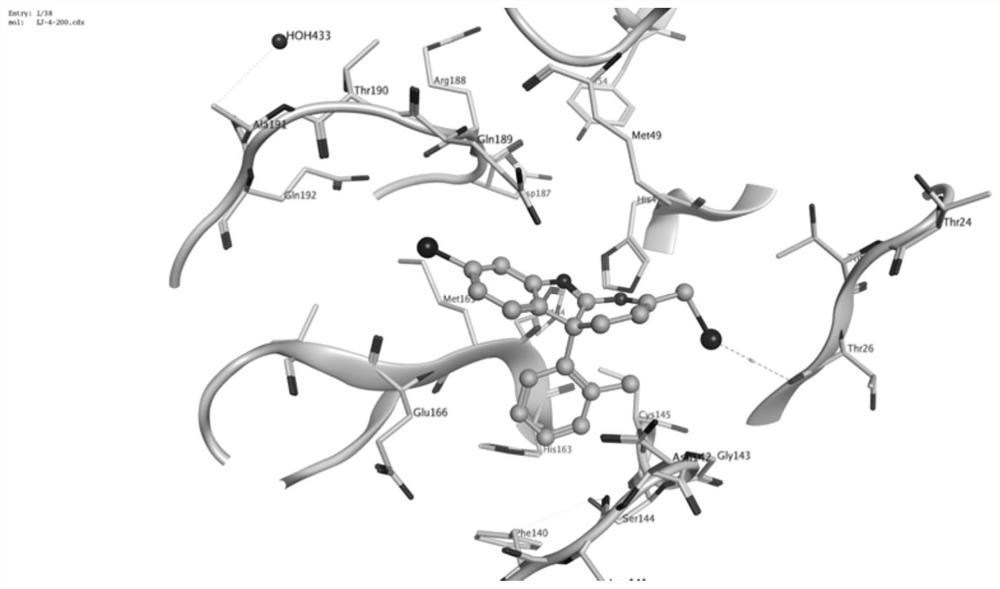
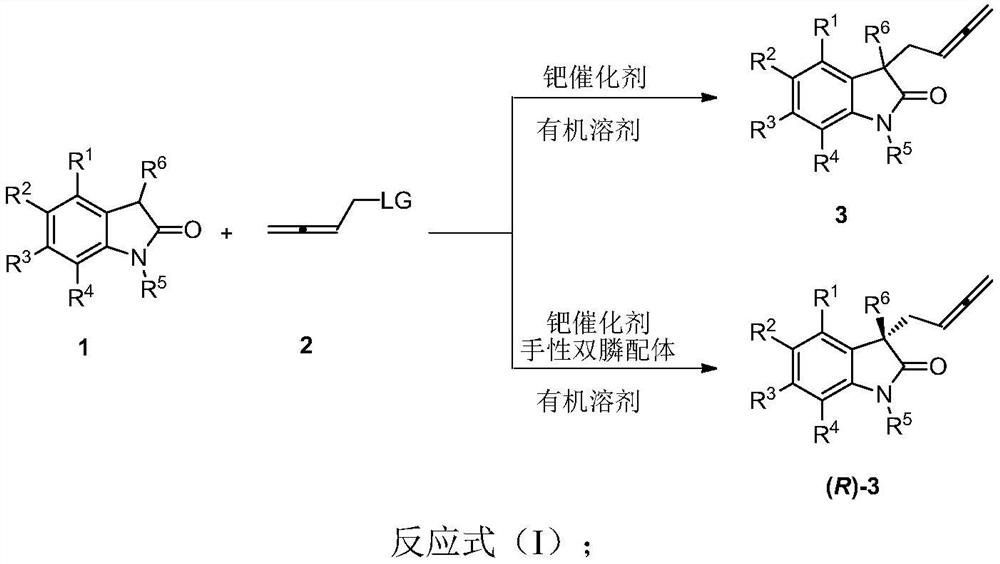
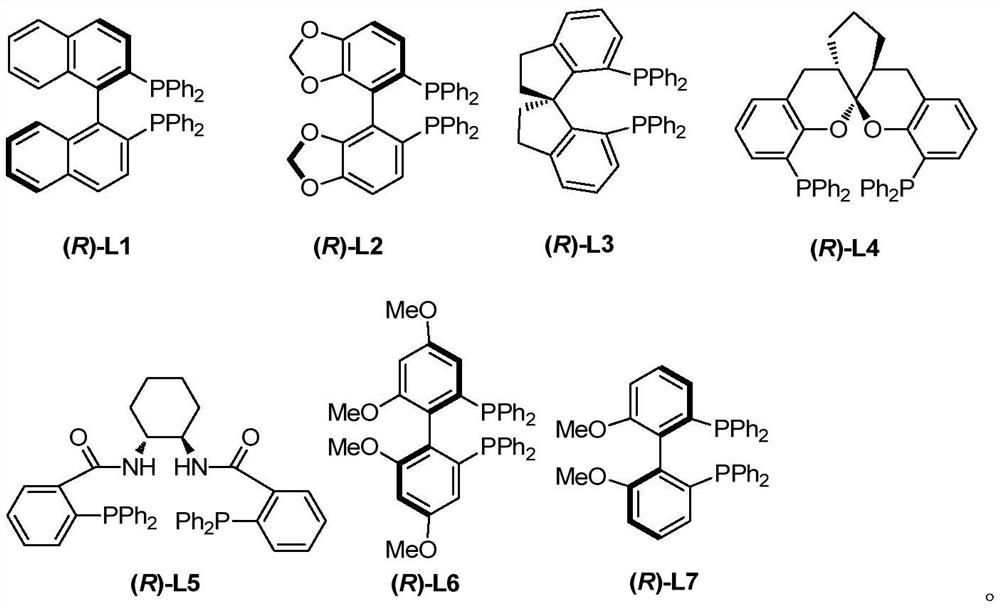
ActiveCN113548999AInhibitory activity hasBroad-spectrum antiviral activityOrganic active ingredientsOrganic chemistry methodsPtru catalystPalladium catalyst
The invention discloses a racemic and chiral 3-(2, 3-butadienyl)oxoindolone compound, a method for preparing the compound by adopting a palladium-catalyzed coupling reaction, and application of the compound. According to the method, 2, 3-butadienyl carbonate and oxoindolone react in an organic solvent in the presence of a palladium catalyst to directly construct the racemic 3-(2, 3-butadienyl)oxoindolone compound in one step. If the palladium catalyst and the chiral phosphine ligand are used in a system and react in then organic solvent, the chiral 3-(2, 3-butadienyl)oxoindolone compound can be directly constructed in one step, and the compound can be easily converted into other complex molecules. The method is convenient to operate, raw materials and reagents are easy to obtain, the substrate universality is wide, the functional group compatibility is good, and the reaction has good conversion rate and high enantioselectivity and chemical selectivity. Besides, the 3-(2, 3-butadienyl)oxoindolone compound and related derivatives thereof provided by the invention can be combined with SRAS-CoV-2 main protein 3CL hydrolase, and have a good application prospect in the aspect of treating human virus infection.
Owner:FUDAN UNIV
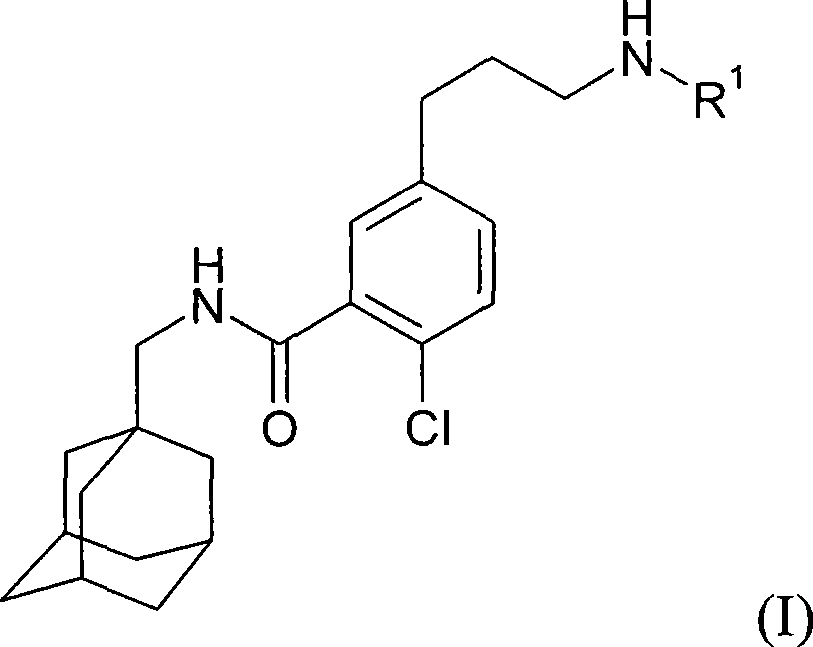
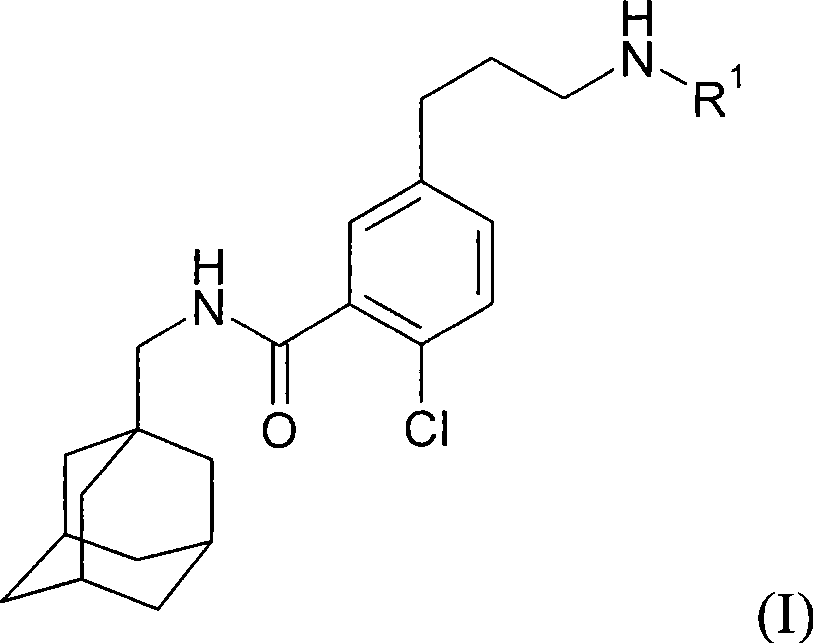
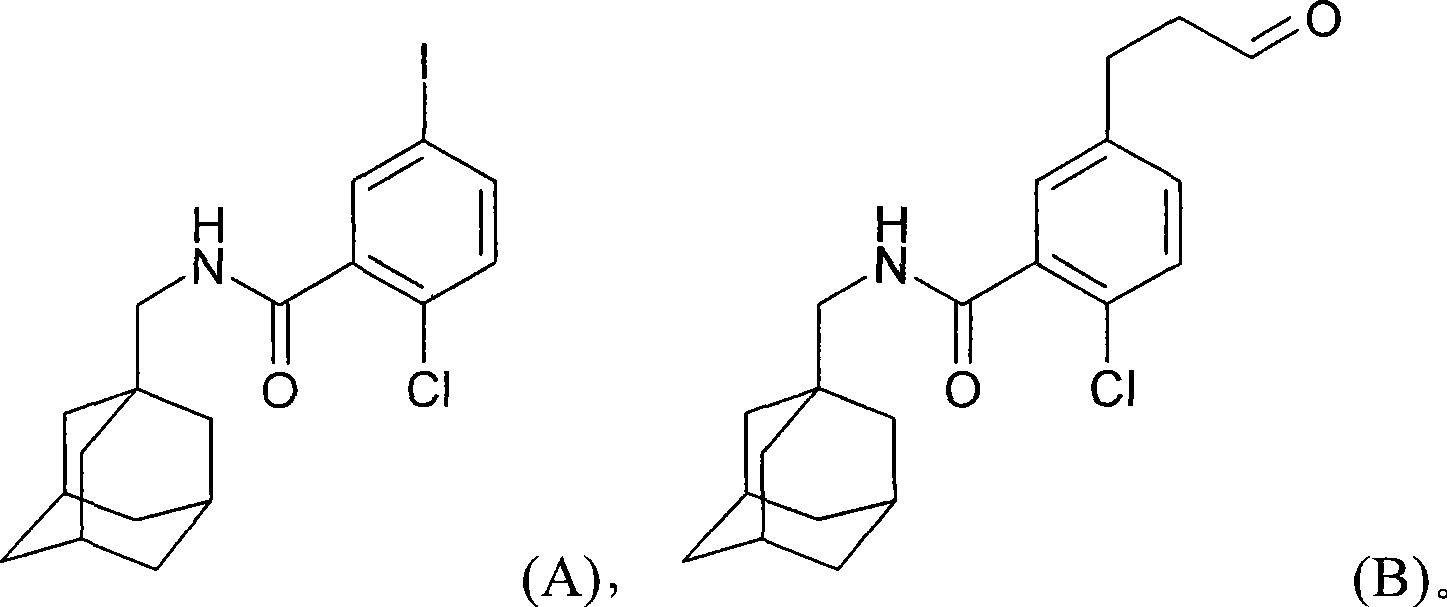
Process for preparing N-methyladamantyl derivatives by a palladium catalysed coupling reaction followed by reductive amination
InactiveCN101511777AOrganic compound preparationCarboxylic acid amides preparationMethyl palmoxirateAmination
The application concerns a process for preparing 2-chloro-5- (3-amino) -N- (methyladamantyl) -benzamide derivatives. The first step of the process involves a palladium catalysed coupling reaction between an iodine containing compound and allyl alcohol with a tertiary amine as base. The second step involves a reductive amination on the formed aldehyde followed by subsequent transformation of the formed amine into a pharmaceutically acceptable salt.
Owner:ASTRAZENECA AB
Method for removing by-product in a palladium-catalyzed coupling reaction in situ
InactiveCN103193573AReduce precipitationReduce concentrationOrganic compounds purification/separation/stabilisationHydrocarbonsPalladium on carbonPorous medium
The invention discloses a method for removing by-product in a palladium-catalyzed coupling reaction in situ. The method disclosed by the invention comprises the following steps of: adding a water-containing porous medium into a reaction system of the palladium-catalyzed coupling reaction and instantly removing by-products in the palladium-catalyzed coupling reaction; and adding an acid-binding agent into the palladium-catalyzed coupling reaction. According to the method disclosed by the invention, by adding the water-containing porous medium into the coupling reaction system, ammonium salt formed in the reaction process is absorbed by the water-containing porous medium, the concentration of the by-product ammonium salt in the reaction system is effectively reduced and the precipitation of the ammonium salt is greatly reduced, so that a palladium carbon catalyst can stably play the role of catalyzing in an organic solution. The method disclosed by the invention is simple and effective, low in cost and easy for industrial production.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Dialkyl(2-alkoxy-6-aminophenyl)phosphine, the preparation method and use thereof
ActiveUS20140309422A1Short routeEasy to operateOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsAlkoxy groupPalladium catalyst
The present invention relates to the compound of dialkyl(2-alkoxy-6-aminophenyl)phosphine and the preparation method thereof and the application in the palladium catalyzed coupling reactions of aryl chloride and ketone. The dialkyl(2-alkoxy-6-aminophenyl)phosphine of the present invention could coordinate with the palladium catalyst to highly selectively activate the inert carbon-chlorine bond, and to catalyze direct arylation reaction in the α-position of ketones to produce corresponding coupling compounds. The preparation method of the present invention is a simple one-step method which produces the air-stable dialkyl(2-alkoxy-6-aminophenyl)phosphine. Compared with the synthetic routes of ligands to be used in the activation of carbon-chlorine bonds in the prior arts, the preparation method of the present invention has the advantages of short route and easy operation.
Owner:ZHEJIANG UNIV
L-alanine derivative preparation method
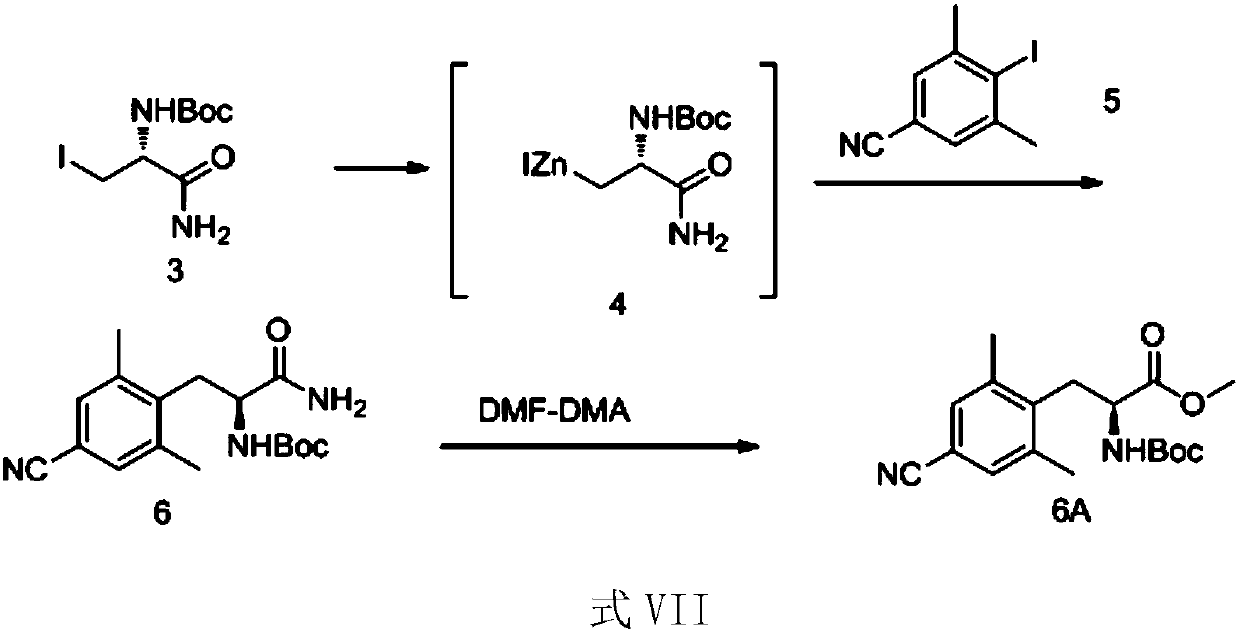
ActiveCN110092735AOvercome the problems of low reaction yield and many by-productsHigh yieldCarbamic acid derivatives preparationOrganic compound preparationEnvironmental resistanceOrganic synthesis
The invention relates to the preparation of pharmaceutical intermediates, and belongs to the field of organic synthesis, particularly to an L-alanine derivative preparation method, which comprises: carrying out a Negishi reaction on a compound 3 to obtain a zinc reagent solution containing an intermediate state compound 4; carrying out a palladium-catalyzed coupling reaction on the zinc reagent solution and a compound 5 to obtain a compound 6; and hydrolyzing the compound 6 to convert into a compound 7. According to the present invention, in the six-step reaction of the route, only the products of the three steps are required to be separately purified, and the products of other steps can be directly poured into the next reaction through the direct one-pot method or after the pressure reducing drying, such that the post-treatment cost of the reaction is substantially saved, the production is accelerated, and the production efficiency is improved; and by using the compound 3 as the starting raw material, the overall yield of the target compound 7 is not less than 46.7%, such that the atomic economy rate is high, the process is environmentally friendly, and the production cost is low.
Owner:SYNCOZYMES SHANGHAI
Preparation method of 4CzIPN type organic polymer and application of 4CzIPN type organic polymer in photocatalytic synthesis
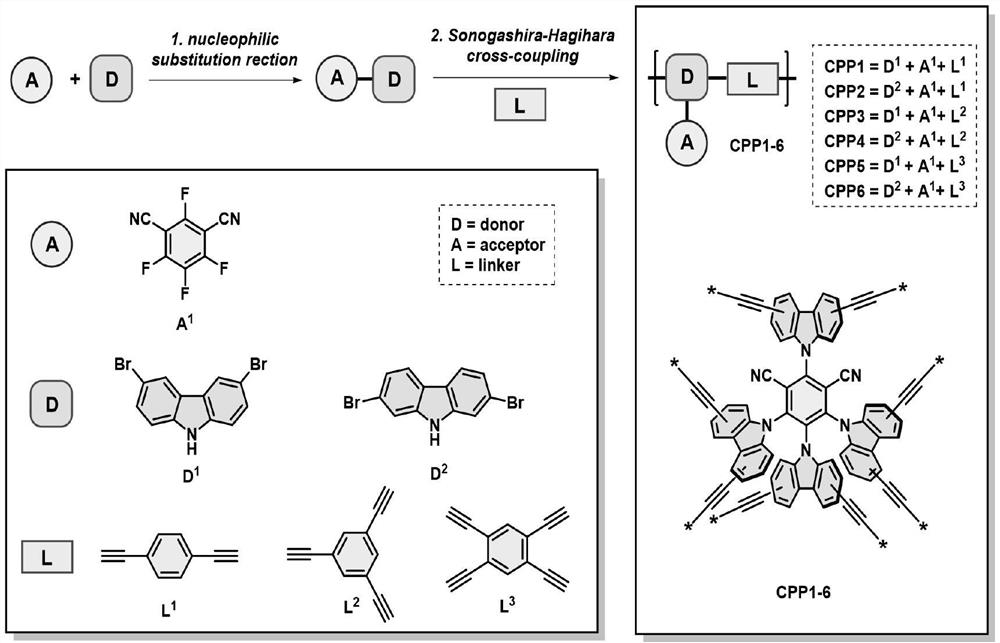
ActiveCN114213633AKeep recyclableHigh catalytic efficiencyOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsBenzeneCarbazole
The invention relates to a preparation method of a 4CzIPN type organic polymer and an application of the 4CzIPN type organic polymer in photocatalytic synthesis, and provides a preparation method of the 4CzIPN type organic polymer based on a 2, 4, 5, 6-tetra (9H-carbazole-9-yl) isophthalonitrile (4CzIPN) fluorescent molecule, which comprises the following steps: taking 2, 4, 5, 6-tetrafluoroisophthalonitrile and 3, 6-dibromo-9H-carbazole or 3, 6-dibromo-9H-carbazole as initial raw materials, and reacting at the temperature of 60-80 DEG C to obtain the 4CzIPN type organic polymer based on the 2, 4, 5, 6-tetra (9H-carbazole-9-yl) isophthalonitrile (4CzIPN) fluorescent molecule. The preparation method comprises the following steps: carrying out a nucleophilic substitution reaction to generate a brominated 4CzIPN monomer in one step, then carrying out a palladium-catalyzed Sonogashira-Hagihara coupling reaction to enable the brominated 4CzIPN monomer to respectively react with 1, 4-phenylenediyne, 1, 3, 5-benzene triyne or 1, 2, 4, 5-benzene tetrayne to sequentially construct six polymers with different skeletons, and taking the synthesized polymers as a heterogeneous photocatalyst to catalyze visible light induced C (sp3)-H bond functionalization reaction, so as to obtain the polymer with the high molecular weight. The method has the advantages of mild conditions, wide substrate application range, greenness, sustainability and the like, and proven that the method has a huge application prospect in organic photochemical reactions.
Owner:XINYANG AGRI & FORESTRY UNIV
A kind of preparation method of avastatin
The invention belongs to the field of organic chemical synthesis and specifically relates to a preparation method of acrivastine. The preparation method comprises the following steps: by taking p-methyl benzoyl hydrazone and alpha-halogenated pyridine as raw materials, carrying out palladium-catalyzed coupling reaction to obtain an intermediate for synthesizing acrivastine; and further preparing acrivastine. By carrying out non-palladium borate-catalyzed coupling reaction, the preparation method is mild in reaction condition; dangerous butyl lithium is not used, so that the risk in plant production is reduced; the reaction is short in time required and high in efficiency; and the raw materials are cheap and easily available and the cost is lowered. The preparation method provided by the invention provides a novel safe, economic and efficient preparation path for preparation of acrivastine.
Owner:CHONGQING HUAPONT PHARMA
Preparation method of Brexpiprazole intermediate and Brexpiprazole intermediate
InactiveCN105732572AAvoid pollutionLow costOrganic chemistryBulk chemical productionPalladium-catalyzed coupling reactionsBrexpiprazole
The invention relates to a preparation method of a Brexpiprazole intermediate and a novel Brexpiprazole intermediate. Raw materials required in the preparation method are low in price and easy to get, and the method is simple in reaction steps and operation. A palladium-catalyzed coupling reaction is not required; the preparation cost is greatly reduced; and heavy metal pollution is avoided. Besides, the intermediate can be obtained just through simple post-treatment, and an overall reaction yield can reach a relatively ideal level.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
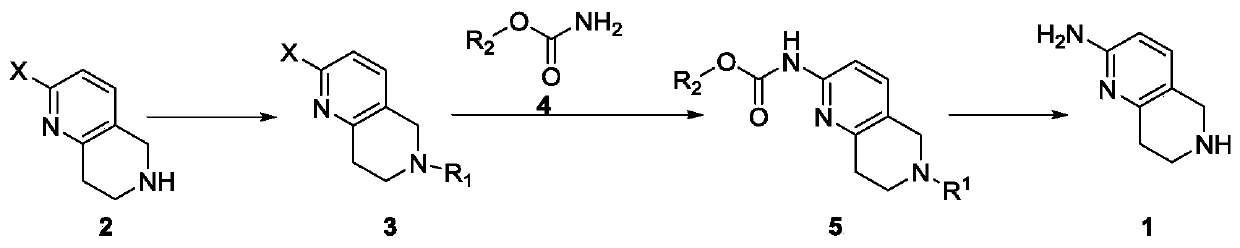
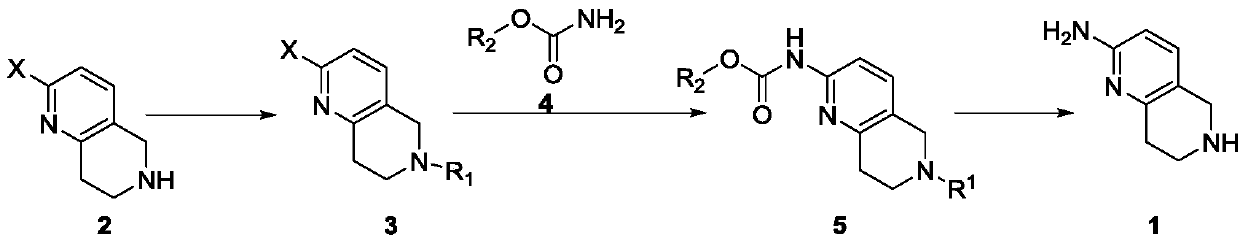
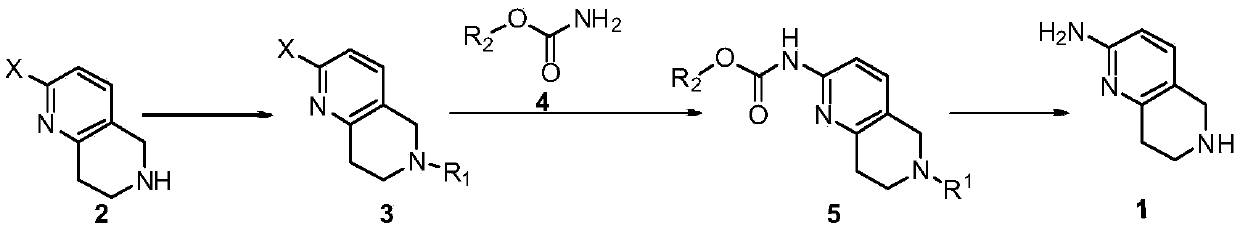
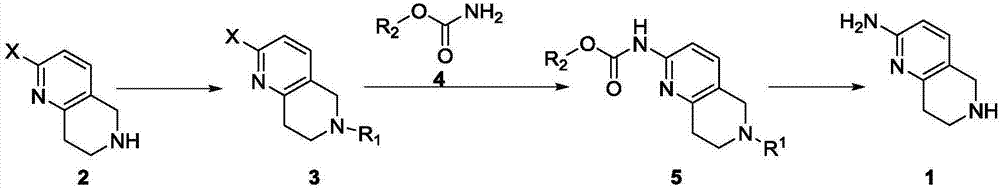
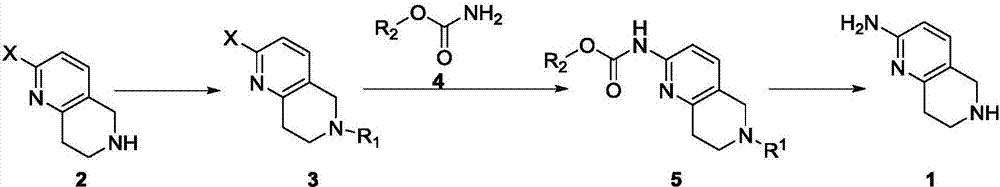
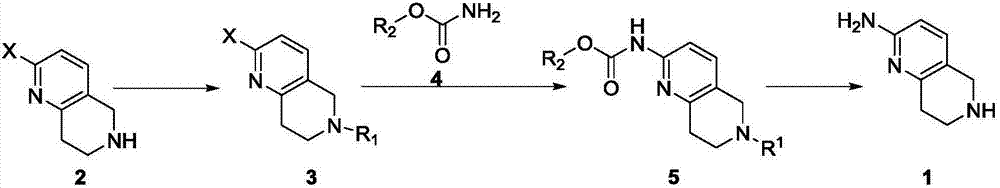
A method for synthesizing 5,6,7,8-tetrahydro-1,6-naphthyridine-2 amine
ActiveCN107021965BMild reaction conditionsOrganic chemistryBulk chemical productionSynthesis methodsPalladium-catalyzed coupling reactions
The invention discloses a synthesis method of 5,6,7,8-tetrahydro-1,6-naphthyridine-2-amine. The synthesis method comprises the specific steps: (1) carrying out amino protection reaction on 2-halo-5,6,7,8-tetrahydro-1,6-naphthyridine to prepare 6-site-protected 2-halo-5,7,8-trihydro-1,6-naphthyridine; (2) carrying out palladium-catalyzed coupling reaction on the 6-site-protected 2-halo-5,7,8-trihydro-1,6-naphthyridine and carbamate under basic conditionsto prepare double-site-protected 5,7,8-trihydro-1,6-naphthyridine-2-amine; (3) carrying out deprotection reaction on the double-site-protected 5,7,8-trihydro-1,6-naphthyridine-2-amine to prepare 5,6,7,8-tetrahydro-1,6-naphthyridine-2-amine. In the whole technical process, only conventional reagents are used, the use of azides with high toxicity and environmental hazards is avoided, reaction conditions are mild and the method is safer and more environmentally friendly.
Owner:CHEMSHUTTLE
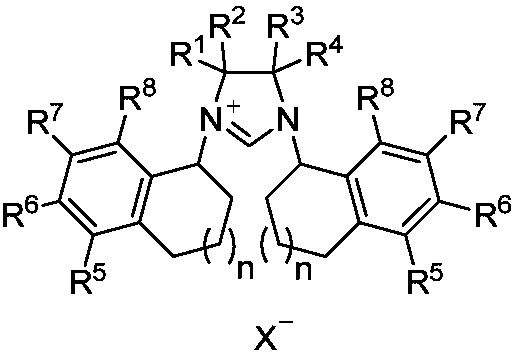
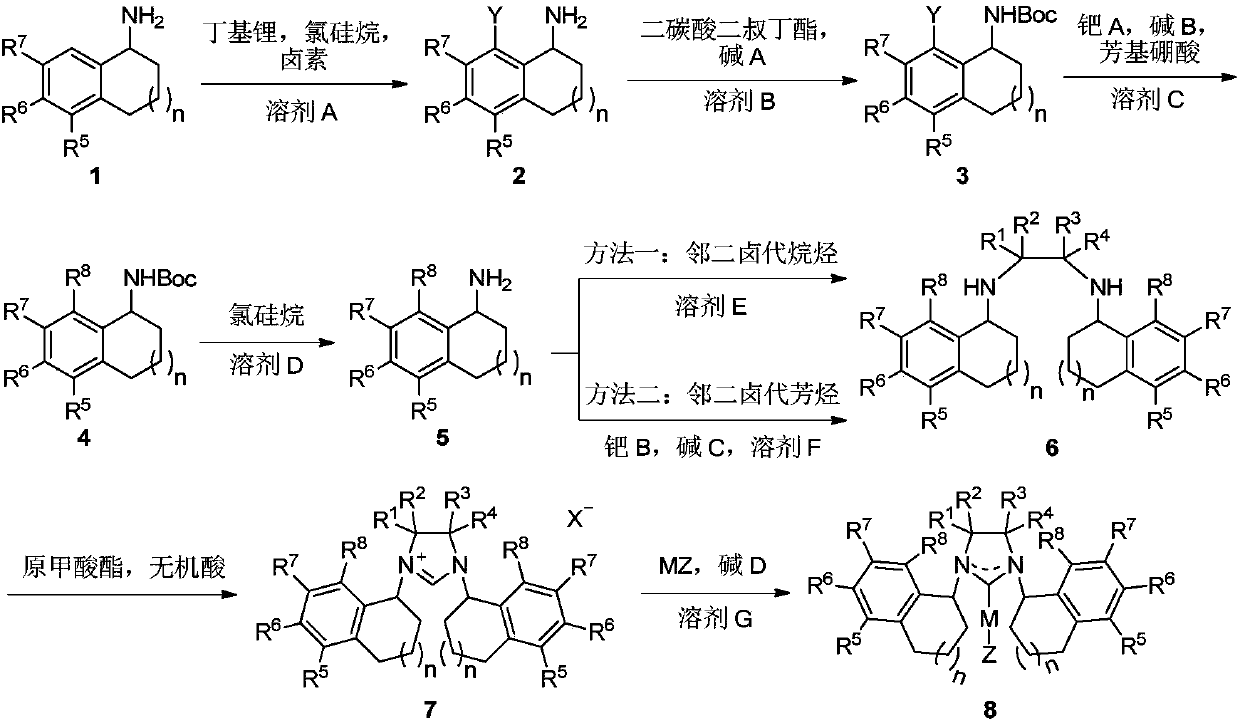
Chiral carbene precursor compound with sandwich structure and synthesis method thereof
ActiveCN110452178AFew reaction stepsHigh yieldOrganic compound preparationOrganic chemistry methodsSynthesis methodsBoronic acid
The invention provides a chiral carbene precursor compound with a sandwich structure. A synthesis method of the compound comprises the following steps: optically pure 1-aminotetralin is used as a starting raw material, is subjected to a palladium-catalyzed coupling reaction to achieve cross-coupling with an aryl boronic acid, then is subjected to a dialkylation reaction with 1,2-dibromoethane, ora coupling reaction with 1,2-dibromobenzene under palladium catalysis, and finally is subjected to a ring-closing reaction with triethyl orthoformate to obtain the chiral carbene precursor compound with the sandwich structure. The optically pure sandwich structure chiral carbene precursor compound can be effectively synthesized, the operation is simple and convenient, the yield is high, and the chiral carbene can effectively realize an asymmetric hydrosilylation reaction of C=O.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
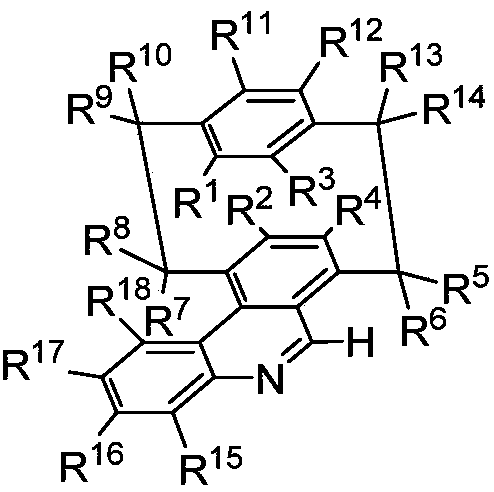
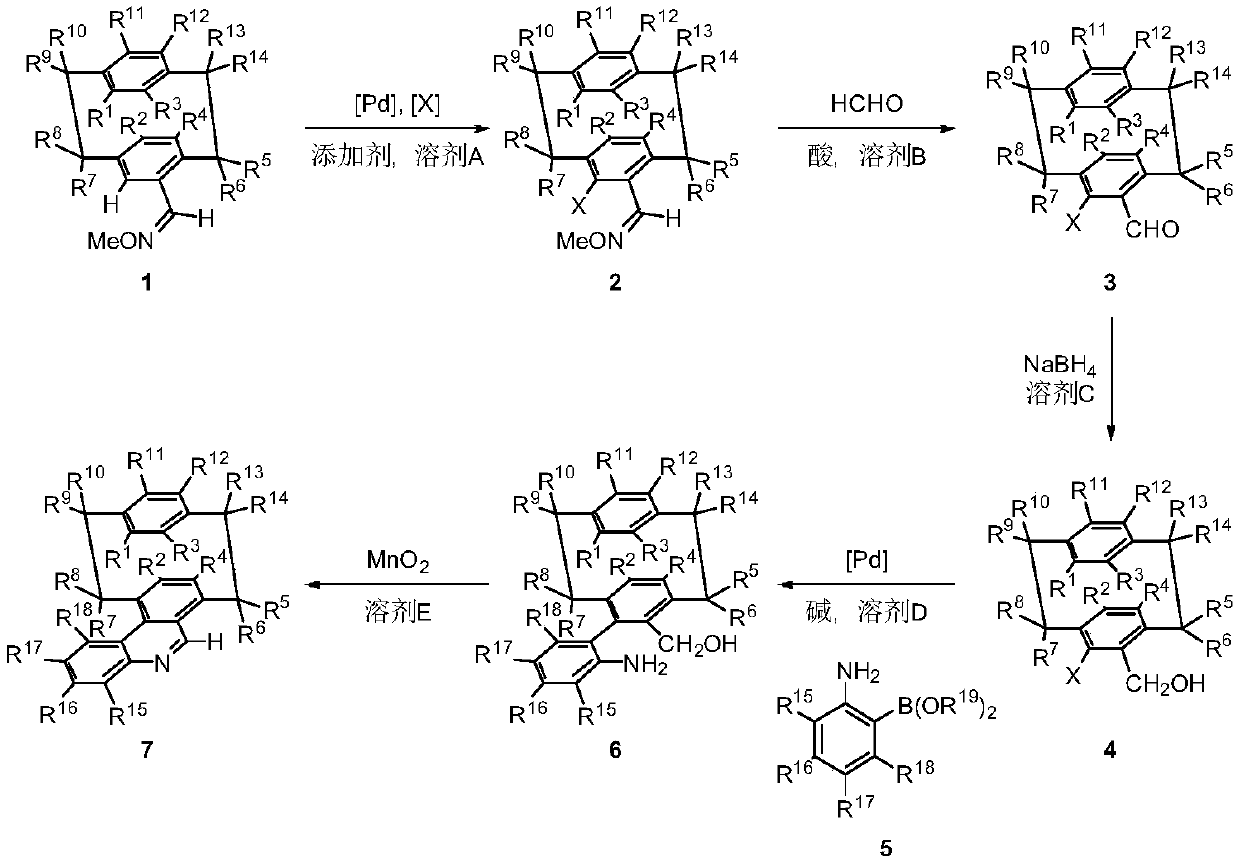
A class of NAD(P)H stimulants with planar chiral cyclophane quinoline skeleton, synthesis method and applications thereof
ActiveCN111116471APromotes oxidative ring closure reactionsAsymmetric hydrogenationOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsStimulantQuinoline
The invention relates to design and synthesis of a class of NAD(P)H stimulants with a planar chiral cyclophane quinoline skeleton. According to the synthesis, optically pure cyclophane formaldoxime ether is used as an initial raw material; o-iodo cyclophane formaldoxime ether can be obtained through a carbon-hydrogen bond activation method; o-iodo cyclophane formaldehyde can be obtained by utilizing an acid-promoted hydrolysis reaction; o-iodo cyclophane methanol can be obtained through a reduction method; o-iodo cyclophane methanol and o-aminophenylboronic acid or boron ester are subjected tocross coupling under a palladium catalyzed coupling reaction; and finally oxidative cyclization is achieved under an oxidation condition so as to obtain a class of NAD(P)H stimulants with the planarchiral cyclophane quinoline skeleton. According to the invention, the optically pure NAD(P)H stimulant with a planar chiral cyclophane quinoline skeleton can be effectively synthesized, and can be used as a recyclable chiral NAD(P)H stimulant under a hydrogen condition to realize asymmetric hydrogenation of C=N, aromatic heterocyclic compounds.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
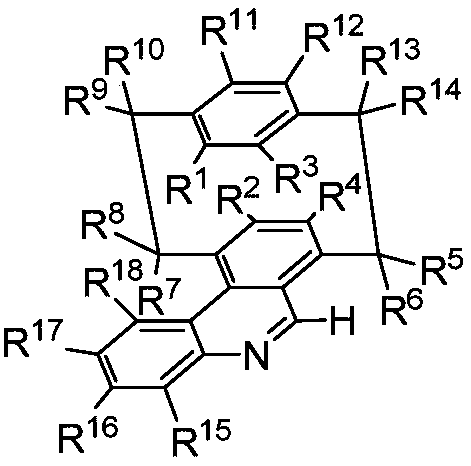
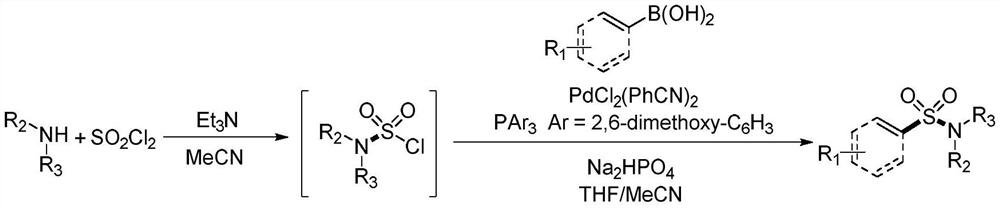
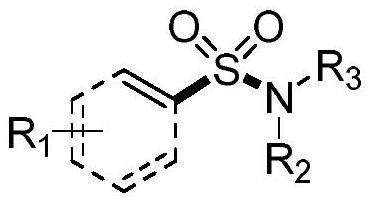
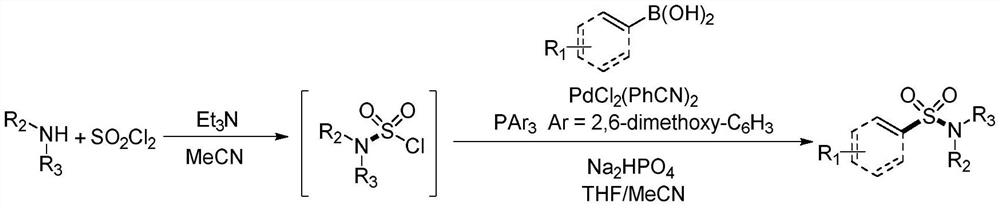
Synthesis method of sulfonyl secondary amine compound
ActiveCN112679292AAvoid cumbersome stepsImprove guidanceSteroidsFunctional group formation/introductionSulfonyl chloridePtru catalyst
The invention belongs to the technical field of organic chemistry, and particularly relates to a preparation method of a sulfonyl secondary amine compound. The structure of the compound is characterized by 1H NMR, 13C NMR and HRMS and is confirmed. According to the method, acetonitrile and tetrahydrofuran are used as a mixed solvent, corresponding secondary amine, sulfonyl chloride and boric acid react under the heating condition in the presence of a palladium catalyst, a ligand and alkali, the secondary amine and the sulfonyl chloride firstly generate corresponding amino sulfonyl chloride, and then the amino sulfonyl chloride and the boric acid are subjected to a palladium-catalyzed Suzuki-Miyaura coupling reaction to obtain the target sulfonyl secondary amine compound. The preparation method of the compound has the advantages that corresponding sulfonyl chloride is prevented from being prepared in advance, meanwhile, the conditions are mild, the compatibility of functional groups is high, separation and purification are convenient, and good application value is achieved.
Owner:FUDAN UNIV
Synthesis method of 5,6,7,8-tetrahydro-1,6-naphthyridine-2amine
ActiveCN107021965AMild reaction conditionsOrganic chemistryBulk chemical productionSynthesis methodsPalladium-catalyzed coupling reactions
The invention discloses a synthesis method of 5,6,7,8-tetrahydro-1,6-naphthyridine-2-amine. The synthesis method comprises the specific steps: (1) carrying out amino protection reaction on 2-halo-5,6,7,8-tetrahydro-1,6-naphthyridine to prepare 6-site-protected 2-halo-5,7,8-trihydro-1,6-naphthyridine; (2) carrying out palladium-catalyzed coupling reaction on the 6-site-protected 2-halo-5,7,8-trihydro-1,6-naphthyridine and carbamate under basic conditionsto prepare double-site-protected 5,7,8-trihydro-1,6-naphthyridine-2-amine; (3) carrying out deprotection reaction on the double-site-protected 5,7,8-trihydro-1,6-naphthyridine-2-amine to prepare 5,6,7,8-tetrahydro-1,6-naphthyridine-2-amine. In the whole technical process, only conventional reagents are used, the use of azides with high toxicity and environmental hazards is avoided, reaction conditions are mild and the method is safer and more environmentally friendly.
Owner:CHEMSHUTTLE
A kind of polymer semiconductor containing bispyridinediazole derivative acceptor and its preparation method and application
ActiveCN107573489BUniversalHigh synthetic yieldSolid-state devicesSemiconductor/solid-state device manufacturingPolymer scienceTri-n-butyltin
The invention belongs to the field of polymer semiconductor materials, and discloses a polymer semiconductor containing a bipyridine diazole derivative receptor and a preparation method and application thereof. A structure of the polymer semiconductor is shown in a formula (I), and the preparation method comprises the following steps: (1) reacting 2-tributyltin-4-alkylthiophene with a dibromobenzimidazole derivative to prepare an intermediate a; (2) reacting the compound a with a bis-butyltin b to obtain a compound c; (3) performing a reaction on the compound c and N-bromosuccinimide to obtainM1; and (4) performing a palladium-catalyzed coupling reaction on the M1 and (E)-bis1,2-(tri-n-butyltin)ethylene to obtain the final product. According to the invention, the synthetic route is simpleand high-efficiency, the raw materials are easy to obtain, the costs are low, the universality is high and the repeatability is good, and a field effect transistor prepared by using the polymer semiconductor as an active layer obtains outstanding bipolar transmission characteristics, and has wide market prospects.
Owner:XIANGTAN UNIV
A class of nad(p)h mimetics with a chiral cycloaryl alkanoline skeleton and its synthesis method and application
ActiveCN111116471BFew reaction stepsSimple and fast operationOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsAlkaneIodide
Design and synthesis of a class of NAD(P)H mimics with a chiral cycloarkanoquinoline skeleton: using optically pure cycloaryl alkanoxime ethers as starting materials, o-iodine can be obtained by carbon-hydrogen bond activation Cycloaryl alkanes aldoxime ethers, acid-promoted hydrolysis can give o-iodocycloaryl carboxaldehydes, followed by reduction methods to give o-iodocycloaranemethanols, followed by palladium-catalyzed coupling reactions with o-aminophenylboronic acids Or cross-coupling of boronic esters, and finally realize oxidative ring closure under oxidative conditions to obtain a class of NAD(P)H mimics with a chiral cycloaryl-alkanoquinoline skeleton. The present invention can effectively synthesize optically pure NAD(P)H mimics of the chiral ring alkane quinoline skeleton, and the NAD(P)H mimics of the chiral ring alkane quinoline skeleton can be synthesized under hydrogen Under certain conditions, it can be used as a recyclable chiral NAD(P)H mimic to realize C=N, asymmetric hydrogenation of aromatic heterocyclic compounds.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Dialkyl(2-alkoxy-6-aminophenyl)phosphine, the preparation method and use thereof
ActiveUS9243011B2Short routeEasy to operateGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsCouplingPalladium catalyst
The present invention relates to the compound of dialkyl(2-alkoxy-6-aminophenyl)phosphine and the preparation method thereof and the application in the palladium catalyzed coupling reactions of aryl chloride and ketone. The dialkyl(2-alkoxy-6-aminophenyl)phosphine of the present invention could coordinate with the palladium catalyst to highly selectively activate the inert carbon-chlorine bond, and to catalyze direct arylation reaction in the α-position of ketones to produce corresponding coupling compounds. The preparation method of the present invention is a simple one-step method which produces the air-stable dialkyl(2-alkoxy-6-aminophenyl)phosphine. Compared with the synthetic routes of ligands to be used in the activation of carbon-chlorine bonds in the prior arts, the preparation method of the present invention has the advantages of short route and easy operation.
Owner:ZHEJIANG UNIV
Preparation method of Crizotinib or deuterated Crizotinib
ActiveCN106496192AStrong complexing abilityReduced activityOrganic chemistry methodsPalladium catalystPalladium-catalyzed coupling reactions
Belonging to the field of pharmaceutical compounds, the invention relates to a preparation method of Crizotinib or deuterated Crizotinib. The preparation method of Crizotinib or deuterated Crizotinib includes: 1) preparation of a formula III compound; 2) preparation of a formula IV compound; and 3) preparation of a formula V compound. The method provided by the invention employs a formula I compound having nitro as the substrate to carry out palladium-catalyzed coupling reaction, nitro does not have lone pair electrons, and nitro and a palladium catalyst have weak complexation, thus reducing the dosage of palladium catalyst and the difficulty of removing palladium catalyst from the product. In addition, the method employs a mixed solvent of low toxicity isopropyl alcohol and water to conduct recrystallization, reduces the solvent residual in bulk drugs, and significantly increases the purification yield.
Owner:BEIJING KYNING BIOSCI +1
Organic electroluminescence ethereal blue optical material, preparation method and application thereof
InactiveCN101270036BFix stability issuesOrganic chemistryOrganic compound preparationFluorescenceLewis acid catalysis
The present invention provides organic small pure blue materials with high stability. The pure blue materials have a structure as shown in formula 1; wherein, R1 stands for Pi-conjugated group; R2 stands for hydrogen, alkyl and alkoxy; and R3 stands for hydrogen, alkyl and alkoxy. The acid-catalyzed Friedel-Crafts ring-closure reaction in the molecules and the high-efficiency palladium-catalyzed coupling reaction solve the problem of molecular synthesis. The compounds have a structure with a spiral ring; the molecules consist of two mutually vertical structural units; thus the larger size andthe branching structure effectively prevent the aggregation of the molecules; and the high-purity blue light can be prepared. The spiral-ring structure with sp<3> hybridized central carbon atoms has relatively high stability; thus the compounds have excellent stability. The compounds can be used as electroluminescent diodes on the light-emitting layer to send pure blue fluorescence, and have high electric and thermal stability.
Owner:PEKING UNIV
A class of chiral carbene precursor compounds with sandwich structure and synthesis method thereof
ActiveCN110452178BFew reaction stepsHigh yieldOrganic compound preparationOrganic chemistry methodsBoronic acidPalladium-catalyzed coupling reactions
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Production method of diaryl alkyne compound
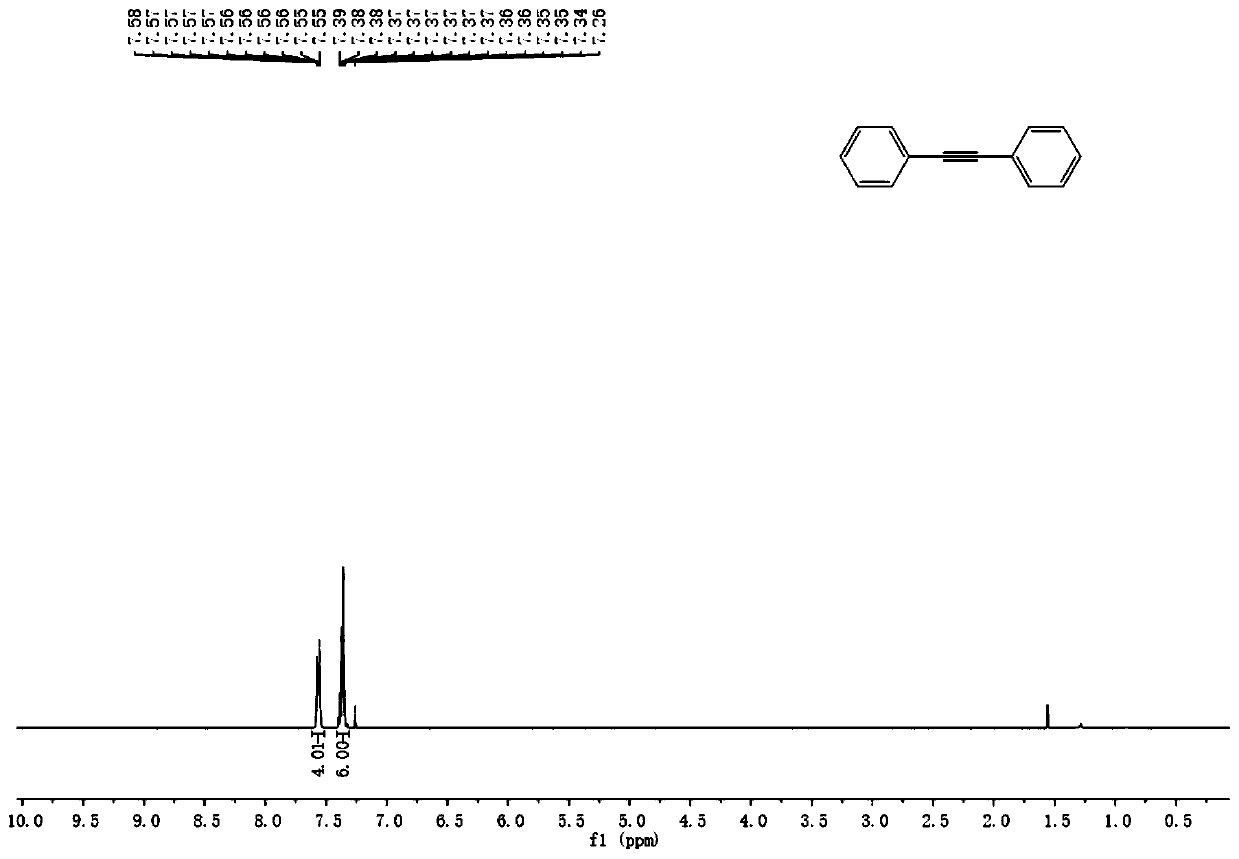
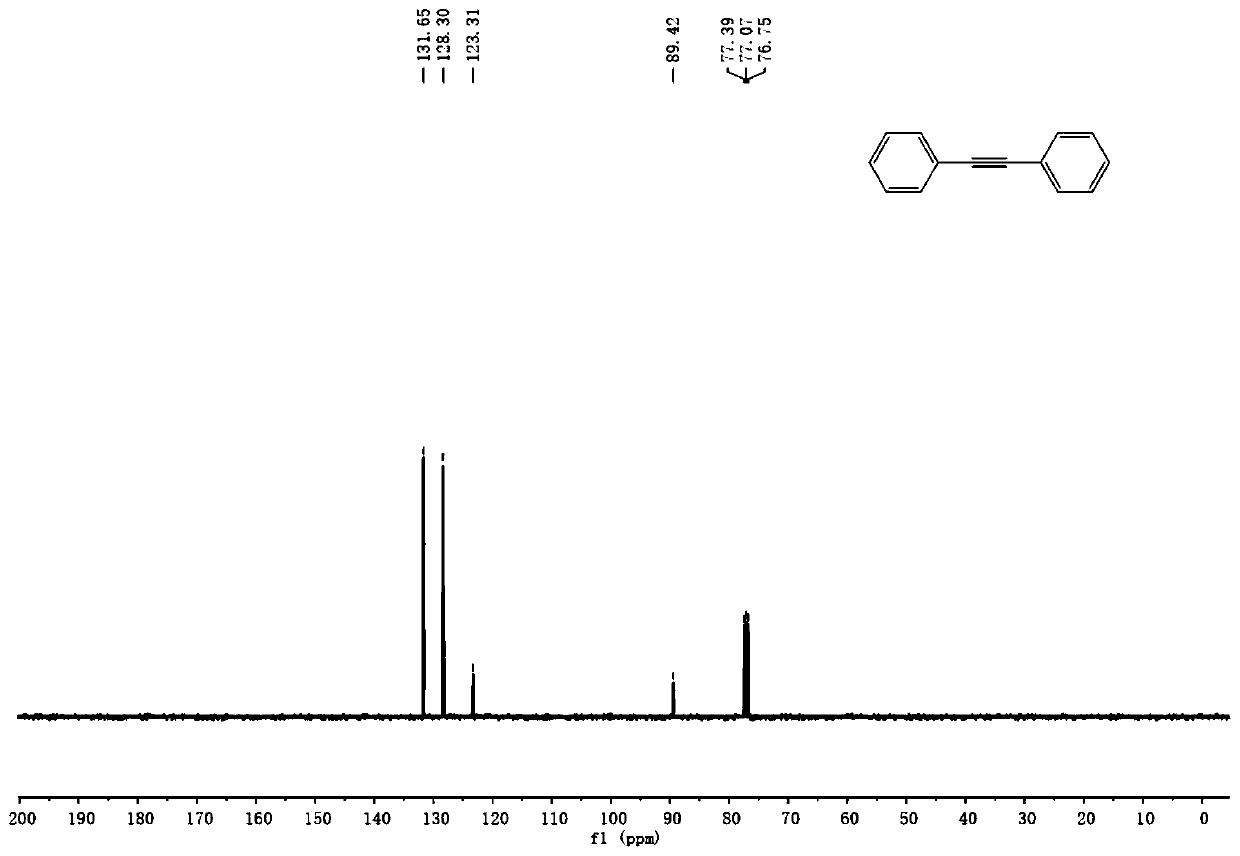
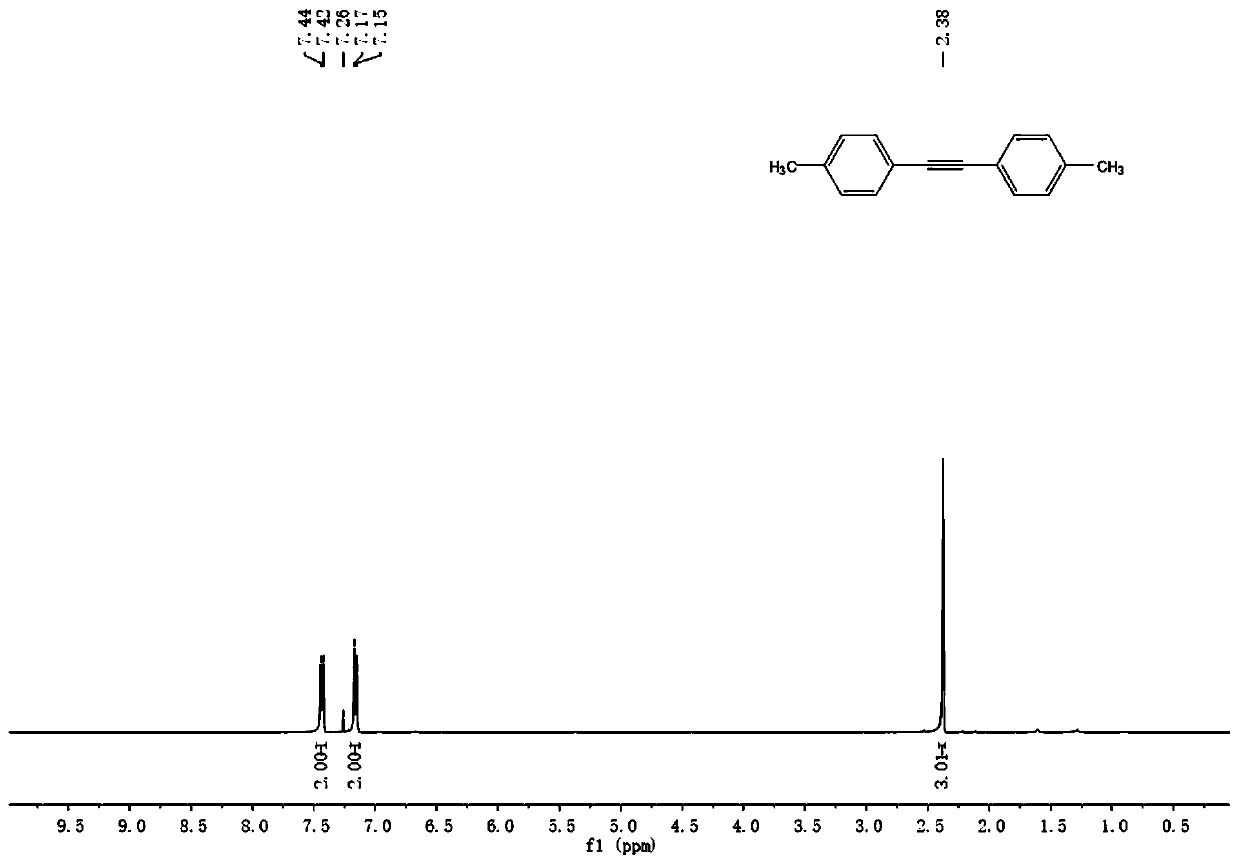
InactiveCN110128231AEasy to prepareRaw materials are easy to obtainAmino preparation from aminesOrganic compound preparationSynthesis methodsPalladium catalyst
The invention discloses a production method of a diaryl alkyne compound. The production method of the diaryl alkyne compound comprises the steps of under protection of nitrogen, placing a bromobenzenesubstrate, alkynol, a palladium catalyst, an organic phosphine ligand and inorganic alkali into a reaction container, then adding a solvent, heating the reaction container for reaction, after the reaction container is cooled to room temperature, adding a saturated ammonium chloride solution for quenching, and then conducting extraction and column chromatography isolation and purification to obtain the diaryl alkyne compound. According to the production method of the diaryl alkyne compound, the aryl bromide and the alkynol which are simple and easy to obtain are used as raw materials, and through the palladium-catalyzed coupling reaction, the diaryl alkyne compound is obtained. The reaction raw materials are simple and easy to obtain, reaction conditions are mild, several defects in a traditional synthesis method are overcome, for example, the defect that when calcium carbide is used as a raw material, the release speed of acetylene cannot be controlled is overcome, the defect that when terminal alkyne is used as a raw material, a product and the raw material are hard to separate due to similar polarity is overcome, and the like. The method also has good performance in amplification reaction, so that the method has great application prospects.
Owner:NANJING UNIV OF POSTS & TELECOMM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com