Preparation method of Crizotinib or deuterated Crizotinib
A technology of deuterium and compound, which is applied in the field of preparation of crizotinib or deuterated crizotinib, can solve the problems of large residual amount of organic solvent and large dosage, and achieve reduction of residual amount and use of organic solvent The effect of reducing the amount and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
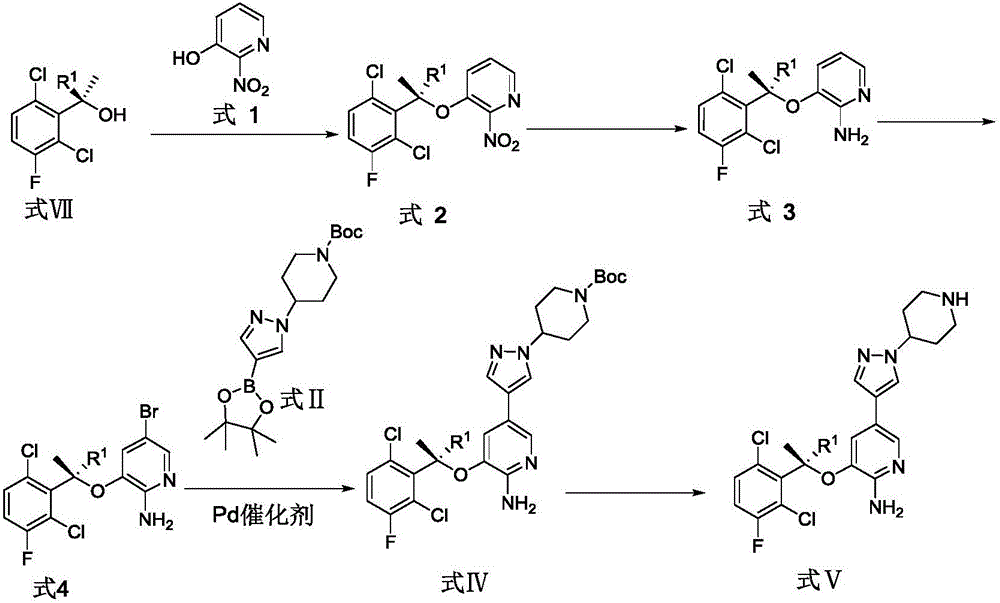
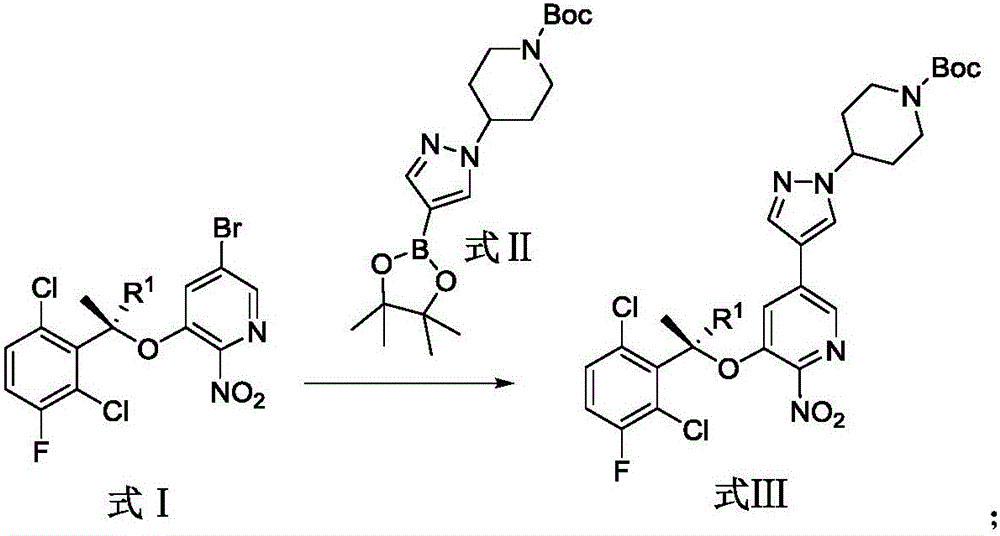
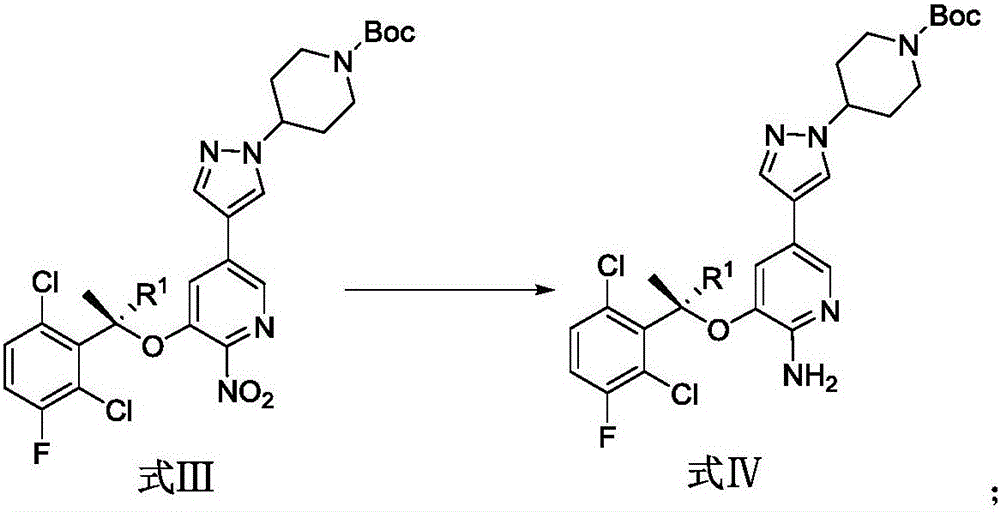
[0050] The synthetic route of crizotinib in the present embodiment is as follows:
[0051]
[0052] In this example, the synthesis of the compound of formula VII and compound of formula II refers to the published patent (publication number: CN104327053A), and the synthesis of the compound of formula VI refers to the patent (WO2012116050A2).
[0053] The synthesis steps of crizotinib in this example are as follows:
[0054] Preparation of formula I compound: compound VII (50g, 238mmol) was added into a 5L three-neck flask containing 1800mL toluene (dried through molecular sieves), N 2 protection, cooling to -5°C; adding PPh 3 (1.1equiv, 262mmol, 68.6g); add compound VI (1.1equiv, 262mmol, 57g) toluene solution 400mL (molecular sieve drying); dropwise add diisopropyl azodicarboxylate (DIAD) (1.1equiv, 262mmol, 52mL ), keep the temperature 1 HNMR (400MHz, CDCl 3 ): 8.09-8.08 (d, J=1.8Hz, 1H), 7.41-7.40 (d, J=1.7Hz, 1H), 7.36-7.32 (dd, J=4.8Hz, 8.9Hz, 1H), 7.14-7.10 (dd, J=...
Embodiment 2
[0060] The synthetic route of deuterated crizotinib in this embodiment is as follows:
[0061]
[0062] In this example, the synthesis of the compound of formula VII and compound of formula II refers to the published patent (publication number: CN104327053A), and the synthesis of the compound of formula VI refers to the patent (WO2012116050A2).
[0063] The synthesis steps of deuterated crizotinib in this example are as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com