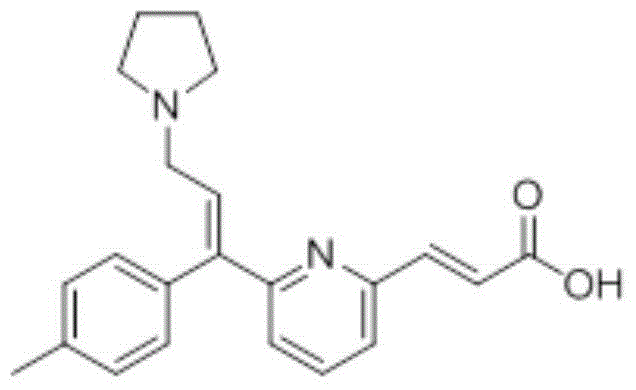
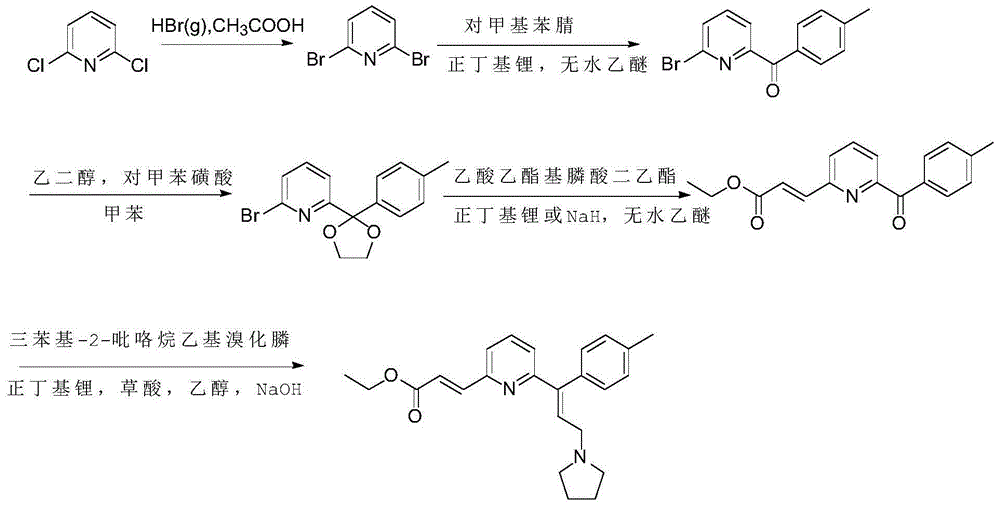
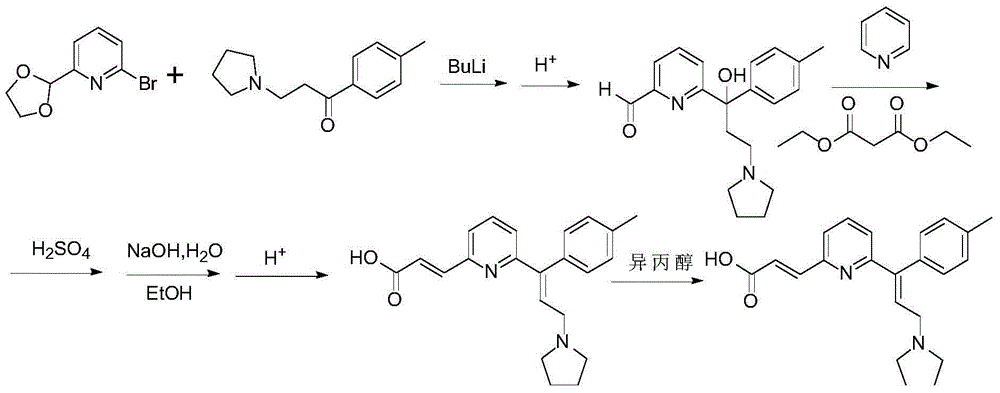
Preparation method of acrivastine
An intermediate, pyrrolidine technology, applied in the field of organic chemical synthesis, can solve problems such as difficult control of industrial batch production, unsatisfactory product yield and purity, and danger, and achieve efficient preparation methods, short time, and reduced risks.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0034] Preparation of Example 14-methyl-N'-(3-(pyrrolidin-1-yl)-1-(p-tolyl)propylene)benzenesulfonylhydrazone
[0035] In a three-necked reaction flask, add 100 g (460 mmol) of 3-(pyrrolidin-1-yl)-1-(p-tolyl)propan-1-one, 102.8 g (552 mmol) of p-toluenesulfonyl hydrazide, and Add 7.9 g (46 mmol) of toluenesulfonic acid, add 120 ml of tetrahydrofuran, stir and reflux, react for 2.5 hours, monitor by TLC spotting, if the reaction is not complete, continue to heat and reflux. After the reaction was completed, the crude product was evaporated to dryness under reduced pressure, then added n-hexane and stirred into a slurry, filtered and dried to obtain 4-methyl-N'-(3-(pyrrolidin-1-yl)-1-(p Tolyl)propylene)benzenesulfonylhydrazone 168.5g, yield 95%.
Embodiment 2
[0036] The preparation of embodiment 2 Avastin
[0037] In a three-necked reaction flask, add 25.0 liters of 4-methyl-N'-(3-(pyrrolidin-1-yl)-1-(p-tolyl)propylene)benzenesulfonylhydrazone prepared in Example 1 g (64.9mmol), (E)-ethyl 3-(6-bromopyridin-2-yl) ethyl acrylate 17.4g (68.1mmol), Pd 2 (dba) 3 1.2g (1.3mmol), Xphos1.2g (2.6mmol), potassium tert-butoxide 9.5g (84.3mmol), stir and mix well, add dry 1,4-dioxane 150ml, pass into N 2 After two gas replacements, continue to feed N 2 protection, the reaction system was heated to 80°C and reacted at this temperature for 5 hours. HPLC and TLC monitor the reaction. After completion, cool to room temperature and filter. The filter cake is washed twice with 25 ml of tetrahydrofuran. 20.7 g of ethyl 3-(pyrrolidin-1-yl)-1-(p-tolyl)prop-1-en-1-yl)pyridin-2-yl)acrylate, with a purity of 98.9% and a yield of 85%.
[0038]In a three-necked reaction flask, add (E)-ethyl 3-(6-((E)-3-(pyrrolidin-1-yl)-1-(p-tolyl)prop-1-ene-1- Base) ...
Embodiment 3
[0039] The preparation of embodiment 3 Avastin
[0040] In a three-necked reaction flask, add 4-methyl-N'-(3-(pyrrolidin-1-yl)-1-(p-tolyl)propylene)benzenesulfonylhydrazone 30.0 prepared in Example 1 g (77.8mmol), (E)-ethyl 3-(6-bromopyridin-2-yl) ethyl acrylate 19.9g (77.8mmol), Pd 2 (dba) 3 4.2g (4.7mmol), Xphos4.4g (9.3mmol), lithium tert-butoxide 18.6g (233.4mmol), stir and mix evenly, add dry 1,4-dioxane 150ml, pass into N 2 After two gas replacements, continue to feed N 2 protection, the reaction system was heated to 90°C and reacted at this temperature for 6 hours. HPLC and TLC monitor the reaction. After completion, cool to room temperature and filter. The filter cake is washed twice with 25 ml of tetrahydrofuran. 23.7g of ethyl 3-(pyrrolidin-1-yl)-1-(p-tolyl)prop-1-en-1-yl)pyridin-2-yl)acrylate, purity 98.7%, yield 81.0% .
[0041] In a three-necked reaction flask, add (E)-ethyl 3-(6-((E)-3-(pyrrolidin-1-yl)-1-(p-tolyl)prop-1-ene-1- Base) pyridin-2-yl) ethyl ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com