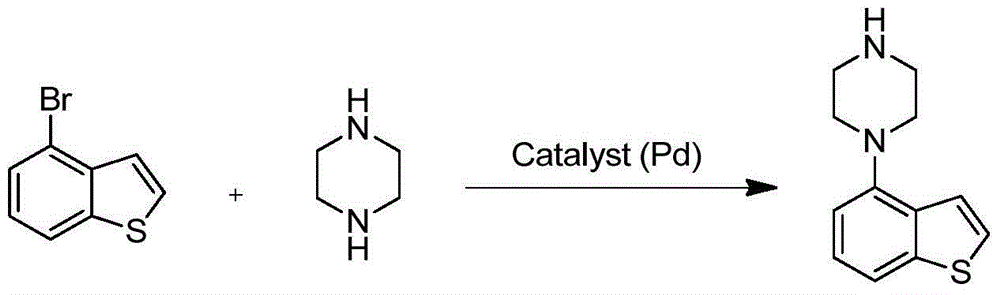
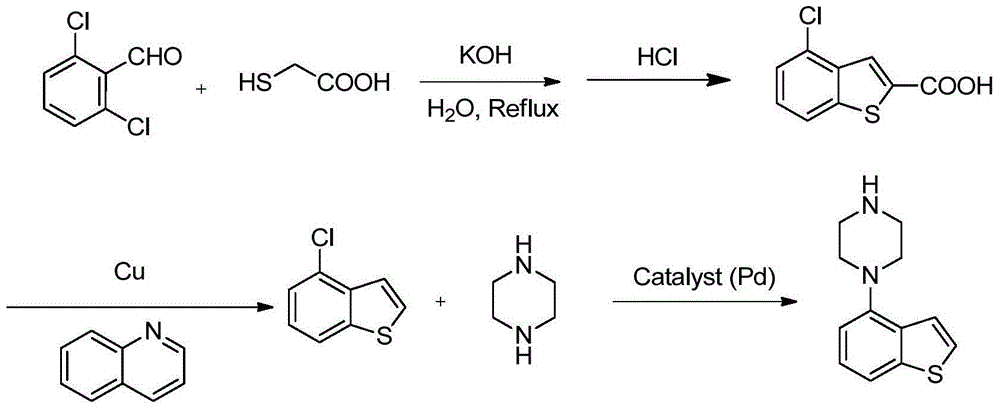
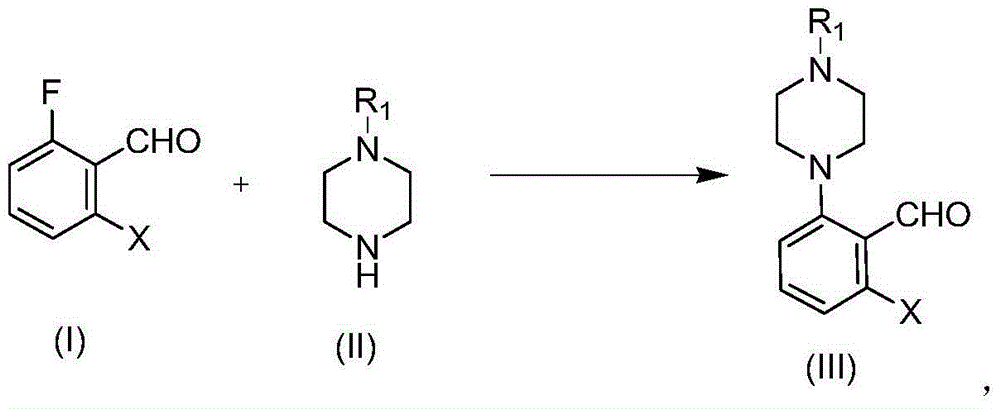
Preparation method of Brexpiprazole intermediate and Brexpiprazole intermediate
A technology for intermediates and compounds is applied in the field of preparation of Brexpiprazole intermediates, which can solve the problems of heavy metal pollution, increase production costs and the like, and achieve the effects of simple operation, cost reduction, and avoidance of heavy metal pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1, the synthesis of 2-(4-acetylpiperazin-1-yl)-6-fluorobenzaldehyde
[0052]
[0053]Add N-acetylpiperazine (6.40g, 50mmol), 2,6-difluorobenzaldehyde (7.46g, 52.5mmol), potassium carbonate (8.28g, 60mmol) and 96mL DMF into a 250mL three-necked flask. The system was heated to 80°C and reacted at this temperature for 18 hours. Stop the reaction, cool to room temperature, add 300mL water, extract three times with ethyl acetate (3×150mL), combine the organic phases, wash with 150mL water, 150mL saturated NaCl solution once, and dry over anhydrous sodium sulfate. After filtration and concentration, the crude product was obtained, which was purified by column chromatography (developer polarity: petroleum ether / ethyl acetate=1 / 2) to obtain 10.21 g of the product, a light yellow solid, with a yield of 82% and a purity of 98%.
[0054] Product NMR data: 1 HNMR (400MHz, DMSO-d 6 )δ10.19(s,1H),7.67–7.55(m,1H),7.00(d,J=8.3Hz,1H),6.94(dd,J=10.7,8.4Hz,1H),3.63(brs,4H...
Embodiment 2
[0055] Embodiment 2, the synthesis of 2-(4-acetylpiperazin-1-yl)-6-chlorobenzaldehyde
[0056]
[0057] 2-Chloro-6-fluorobenzaldehyde (1.00g, 6.3mmol), potassium carbonate (1.74g, 12.58mmol), N-acetylpiperazine (0.89g, 6.92mmol) and 12mL DMF were added to a 25mL single-necked bottle. Stir for 5min. The reaction solution was heated to 100° C. for 15 hours. Stop the reaction, cool to room temperature, add 80 mL of water, extract three times with ethyl acetate (40 mL×3), combine the organic phases, wash with 40 mL of water and 40 mL of saturated NaCl solution once, and dry over anhydrous sodium sulfate. After filtration and concentration, the crude product was obtained, which was purified by column chromatography (developer polarity: petroleum ether / ethyl acetate=1 / 1) to obtain 1.40 g of a yellow solid with a yield of 84% and a purity of 99%.
[0058] Product NMR data: 1 HNMR(400MHz,DMSO)δ10.24(s,1H),7.55(t,J=8.1Hz,1H),7.21(d,J=2.5Hz,1H),7.19(d,J=2.9Hz,1H) ,3.68–3.54(m,4H)...
Embodiment 3
[0059] Embodiment 3, the synthesis of 2-(4-Boc piperazin-1-yl)-6-fluorobenzaldehyde
[0060]
[0061] Add 2,6-difluorobenzaldehyde (4.00g, 28.2mmol), N-Boc piperazine (4.98g, 26.8mmol), potassium carbonate (4.44g, 32.2mmol) and 60mL DMF into a 100mL three-necked flask. The system was heated to 80°C for 15 hours. Stop the reaction, cool to room temperature, pour the reaction solution into 200mL water, extract three times with ethyl acetate (80mL×3), combine the organic phases, wash once with 60mL water, 60mL saturated NaCl solution, and dry over anhydrous sodium sulfate. After filtration and concentration, the crude product was obtained, which was purified by column chromatography (developer polarity: petroleum ether / ethyl acetate=20 / 1) to obtain 5.81 g of a yellow solid with a yield of 70% and a purity of 98%.
[0062] Product NMR data: 1 HNMR (400MHz, CDCl 3 )δ10.32(s,1H),7.47(td,J=8.3,6.4Hz,1H),6.87–6.73(m,2H),3.75–3.56(m,4H),3.12–2.96(m,4H) ,1.49(s,9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com