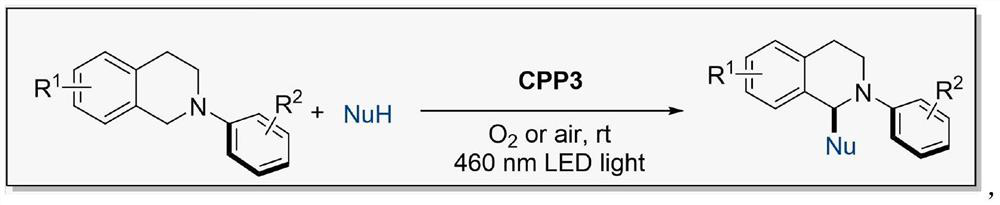
Preparation method of 4CzIPN type organic polymer and application of 4CzIPN type organic polymer in photocatalytic synthesis
A polymer and photocatalyst technology, applied in the field of material chemistry, can solve the problems of poor structural stability, easy photobleaching, high price, etc., achieve mild conditions, high catalytic efficiency, and overcome the effect of high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The present invention will be further described in detail below through the specific examples, the following examples are only descriptive, not restrictive, and cannot limit the protection scope of the present invention with this.
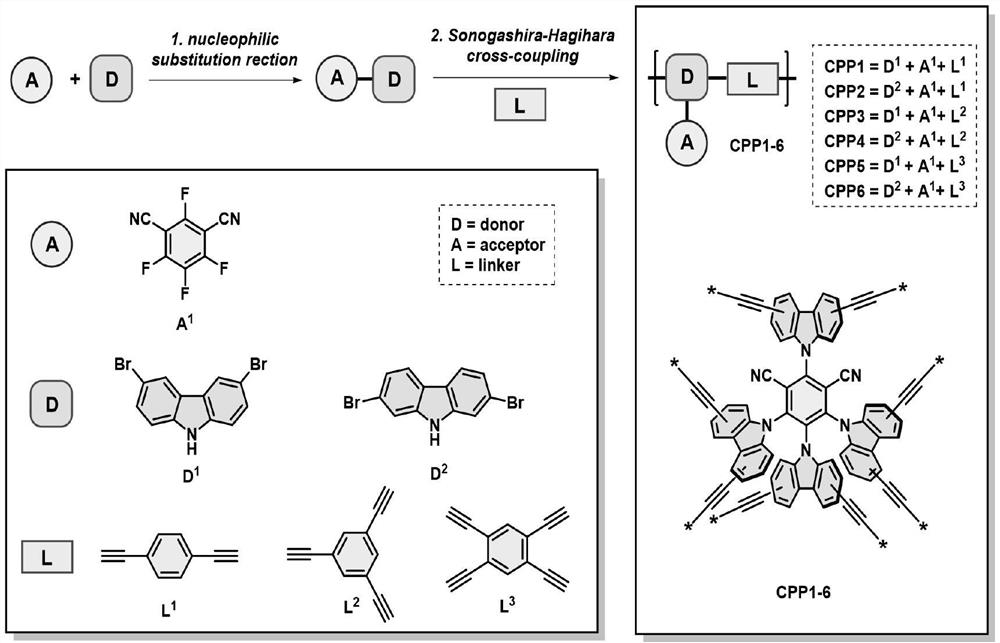
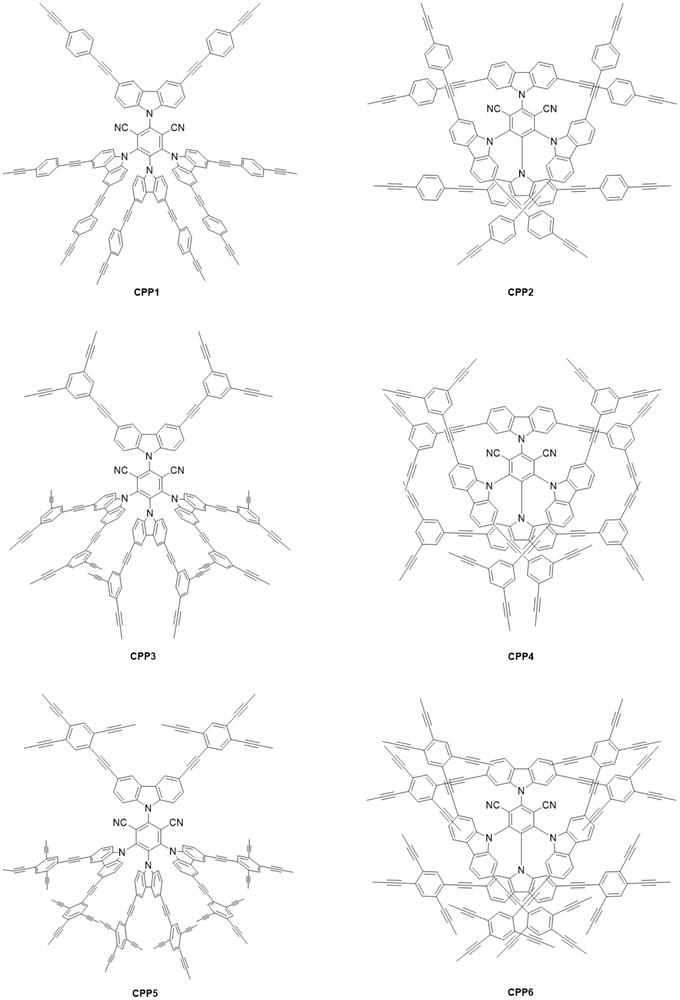
[0020] A preparation method of 4CzIPN polymer CPP3, the steps are as follows:
[0021] In a nitrogen atmosphere, 9.5 g of 3,5-dibromocarbazole was added to 60 ml of dry tetrahydrofuran, and after it was completely dissolved, 1.0 g of sodium hydride was slowly added. After stirring for 30 minutes, 130 mg of 2,4,5,6-tetrafluoroisophthalonitrile was added to the reaction system, and stirring was continued at room temperature for 12 hours. Subsequently, 2 ml of water was added to quench the reaction, and after the solvent was spin-dried, the residue was recrystallized from n-hexane / dichloromethane to obtain 3,6-bromo-4CzIPN as a yellow solid. Add 564 mg of 3,6-bromo-4CzIPN, 12 mg of bistriphenylphosphinepalladium dichloride, 6 mg of ketone iodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com