L-alanine derivative preparation method
A technology of derivatives and alanine, applied in the field of preparation of L-alanine derivatives, can solve the problems of high production cost of intermediates, low chiral purity of products, complicated operations, etc., and achieves improved yield and reduced processing cost, the effect of speeding up production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation of embodiment 1 compound 2
[0053]
[0054] Dissolve compound 1 (22g, 0.1mol) in 220g of methanol, pass ammonia gas into the above mixture until it is saturated, place it in a polytetrafluoroethylene-lined tank and heat it to 80°C for 24 hours, stop heating after detecting that the reaction is complete , cooled to room temperature, the reaction solution was decompressed to remove the solvent, and the obtained product was directly used in the next step without further purification.
Embodiment 2
[0055] The preparation of embodiment 2 compound 3
[0056]
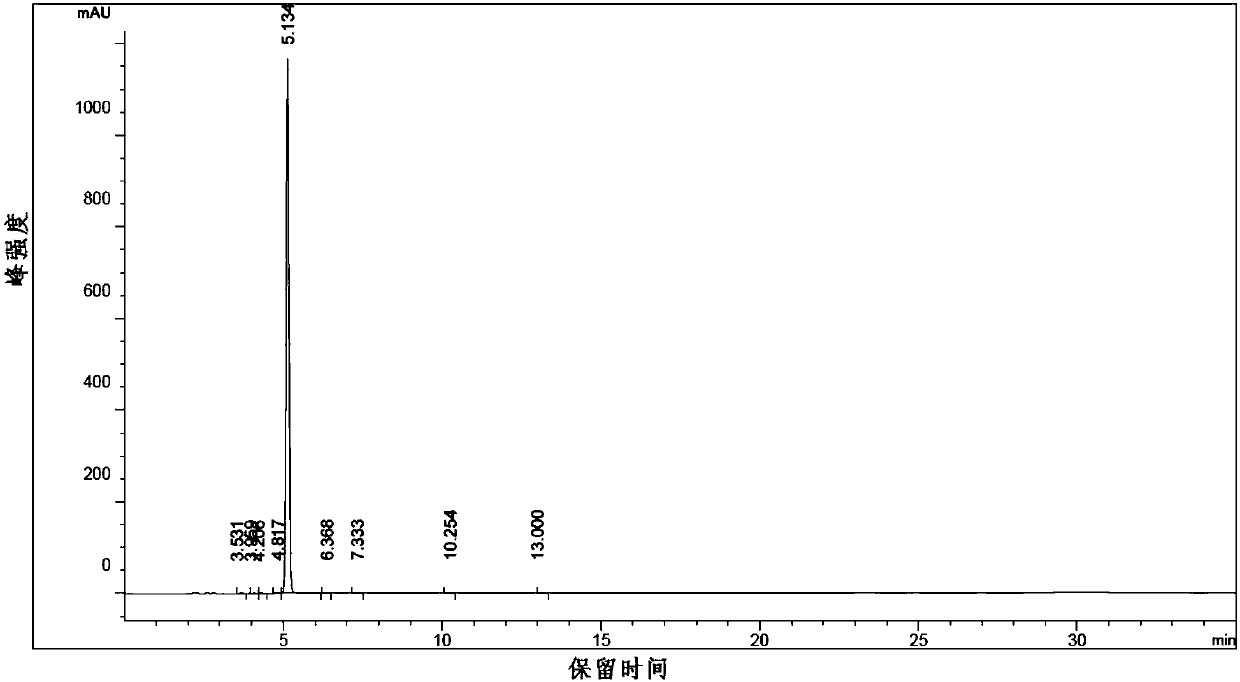
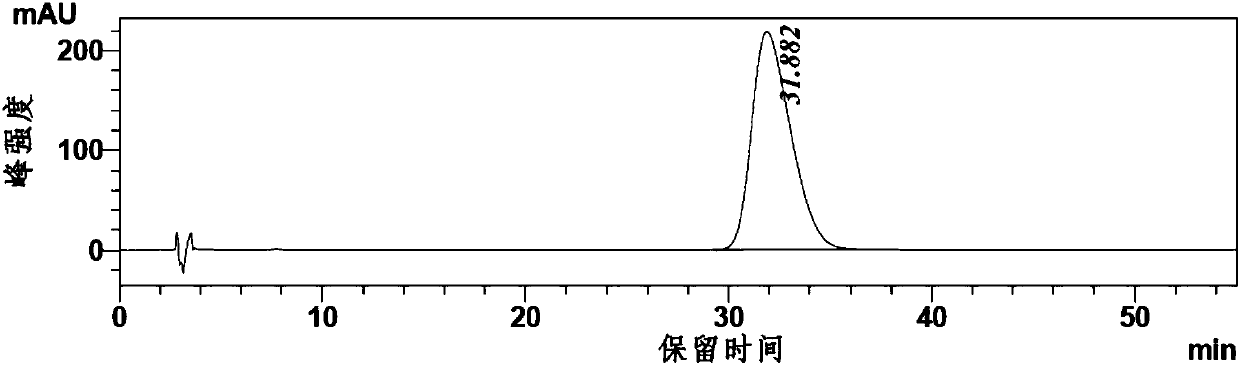
[0057] Dissolve triphenylphosphine (26.2g, 0.1mol) and imidazole (6.8g, 0.1mol) in 340ml of dichloromethane, add iodine (25.4g, 0.1mol) into the above system in three batches, and stir at room temperature for 10 minute, cooled to 0°C, dissolved the crude product obtained in Example 1 in 150mL of dichloromethane and dropped it into the above system within 30 minutes, kept stirring at 0°C for 1h, raised to room temperature and stirred for 1.5h, added 200mL of water, and slowly added 1M hydrochloric acid, The pH value was adjusted to 4, the liquid was separated, the organic phase was concentrated to about 80 mL, and the product compound 3 (24.5 g, 0.078 mol) was obtained by silica gel column chromatography (ethyl acetate: petroleum ether 3: 1). The step yield is 78%.
Embodiment 3
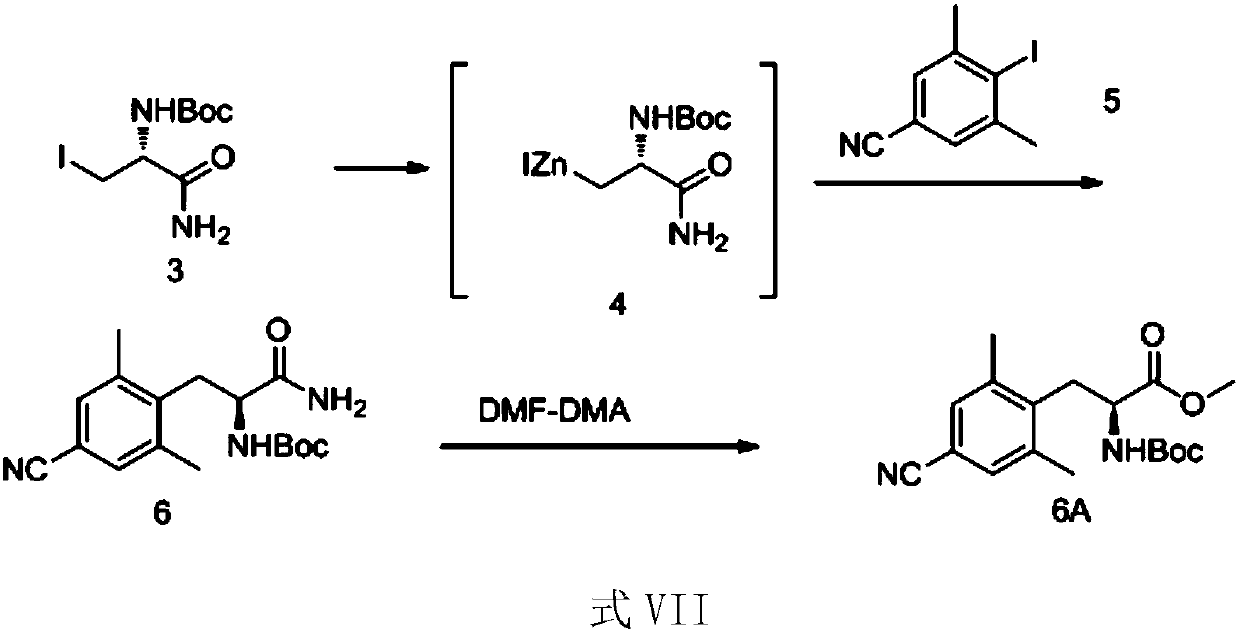
[0058] The preparation of embodiment 3 compound 6
[0059]
[0060]Zinc powder (64 g, 1 mol) and 196 ml of N,N-dimethylformamide were added into the reaction flask, and nitrogen was replaced three times. Add 1,2-dibromoethane (13.9 g, 0.074 mol), raise the temperature of the reaction solution to 85±2° C., and keep the temperature for ten minutes. Cool in an ice bath to 25±2°C, add trimethylchlorosilane (4.8 g, 0.044 mol), and keep warm at 25±2°C for 10 minutes. The reaction solution was cooled to 10-15°C, and volatile substances were removed under reduced pressure. Compound 3 (24.5 g, 0.078 mol) prepared in Example 2 was dissolved in DMF (196 ml) to form a solution. The solution was added dropwise to the reaction solution at 20° C. under temperature control. Stir for 2 hours after the dropwise addition, prepare the zinc reagent and refrigerate for future use.
[0061] Add 2,5-dimethyl-4-cyanoiodobenzene (20.6g, 0.08mol) and P(o-tol) successively in the four-neck flask ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com