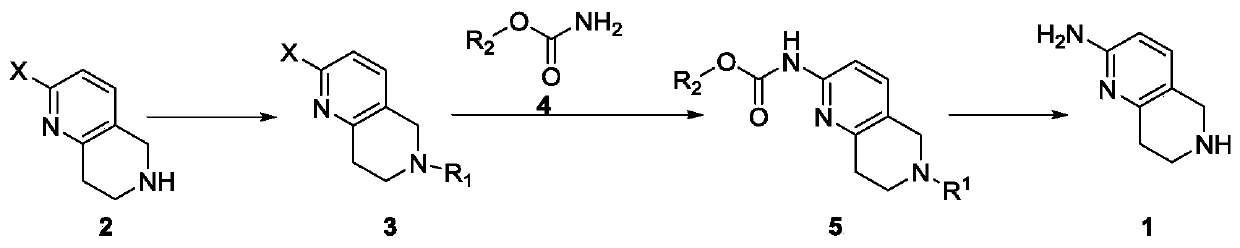
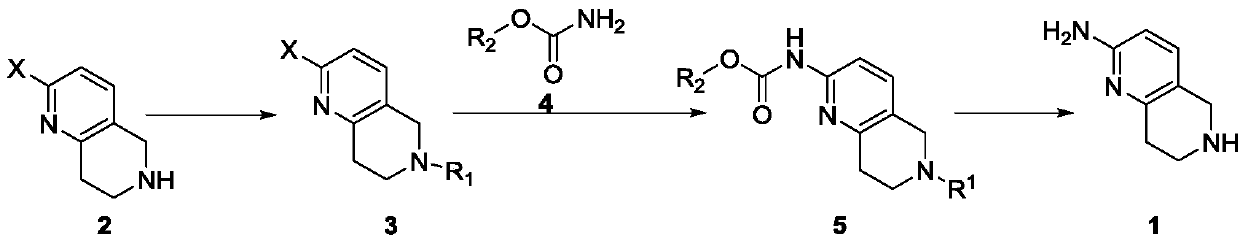
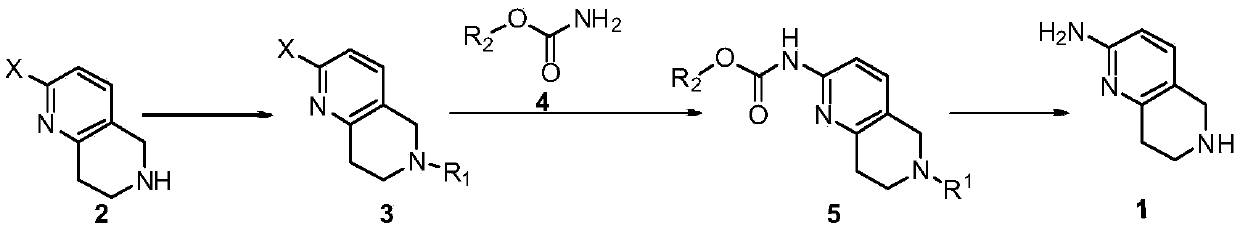
A method for synthesizing 5,6,7,8-tetrahydro-1,6-naphthyridine-2 amine
A synthesis method and naphthyridine technology are applied in the field of synthesis of pharmaceutical intermediates, which can solve the problems of explosion, high environmental hazard, strong toxicity of azide and the like, and achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A method for synthesizing 5,6,7,8-tetrahydro-1,6-naphthyridine-2 amine, said method comprising the steps of:
[0023] (1) In a 250mL single-necked bottle, dissolve 20.0g of 2-chloro-5,6,7,8-tetrahydronaphthyridine and 14.0g of triethylamine in 50mL of dichloromethane, and stir the solution in an ice-salt bath Cool and add 27.2g Boc dropwise 2 O, after the dropwise addition, react at room temperature for 1 hour, add 50 mL of dichloromethane to dilute, wash twice with 1 mol / L dilute hydrochloric acid, once with saturated sodium bicarbonate and once with saturated brine, and dry the organic phase with anhydrous magnesium sulfate , filtered, and the filtrate was spin-dried to obtain 30.9g of a light yellow substance, namely 2-chloro-6-tert-butoxycarbonyl-5,7,8-trihydro-1,6-naphthyridine, yield 97%, LCMS ( ESI):m / z 269(M+H) + ,169(M-100) + .
[0024] (2) Weigh 7.0 g of 2-chloro-6-tert-butoxycarbonyl-5,7,8-trihydro-1,6-naphthyridine prepared in step (1) into a 250 mL sing...
Embodiment 2
[0027] A method for synthesizing 5,6,7,8-tetrahydro-1,6-naphthyridine-2 amine, said method comprising the steps of:
[0028] (1) In a 250 mL single-necked bottle, add 5.0 g of 2-bromo-5,6,7,8-tetrahydronaphthyridine, 6.5 g of potassium carbonate, 5.9 g of benzyl chloride and 50 mL of acetonitrile in sequence. Heat to reflux to react overnight, filter after the reaction is complete, spin the filtrate to remove the solvent and pass through the column, rinse with EA:PE=1:20-1:10 to obtain 6.3g of white solid, namely 2-bromo-6-benzyl-5, 7,8-Trihydro-1,6-naphthyridine, yield 89%, LCMS (ESI): m / z 303, 305 (M+H) + .
[0029] (2) Weigh 5.0 g of 2-bromo-6-benzyl-5,7,8-trihydro-1,6-naphthyridine prepared in step (1) into a 100 mL single-necked bottle, add 50 mL of DMF and stir to dissolve, Then add 3.7g benzyl carbamate, 3.7g Xantphos, 6.8g K 2 CO 3 , and 3.5g tetrakistriphenylphosphine palladium, the reaction mixture was heated to 120°C for 4h under the protection of nitrogen, then...
Embodiment 3
[0032] A method for synthesizing 5,6,7,8-tetrahydro-1,6-naphthyridine-2 amine, said method comprising the steps of:
[0033] (1) In a 250mL single-necked bottle, dissolve 10.0g of 2-chloro-5,6,7,8-tetrahydronaphthyridine and 9.2g of DIEA in 50mL of THF, cool to -10~-5°C, and add 11.1 g benzyl chloroformate, react at room temperature for 2h after the dropwise addition. After the reaction is complete, dilute with 200 mL of water, extract three times with 200 mL of ethyl acetate, wash the organic phase with 1 mol / L dilute hydrochloric acid, saturated sodium bicarbonate and saturated brine, dry over anhydrous magnesium sulfate, filter, and spin the filtrate to Dry to obtain 16.3g of light yellow substance, namely 2-chloro-6-benzyloxycarbonyl-5,7,8-trihydro-1,6-naphthyridine, yield 91%, LCMS (ESI): m / z 303,305(M+H) + .
[0034] (2) Weigh 3.0 g of 2-chloro-6-benzyloxycarbonyl-5,7,8-trihydro-1,6-naphthyridine prepared in step (1) into a 100 mL stuffy tank, add 50 mL THF and stir t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com