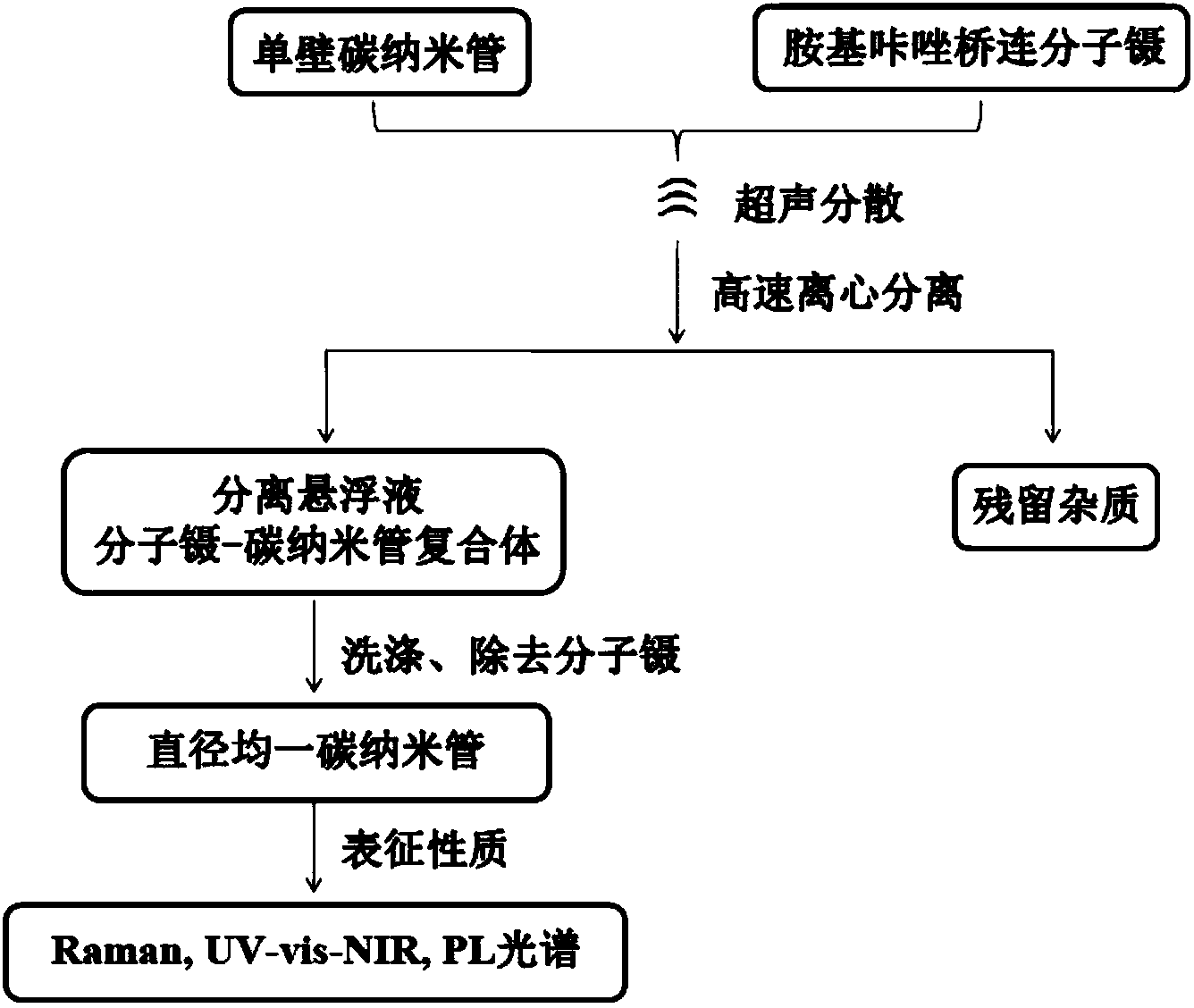
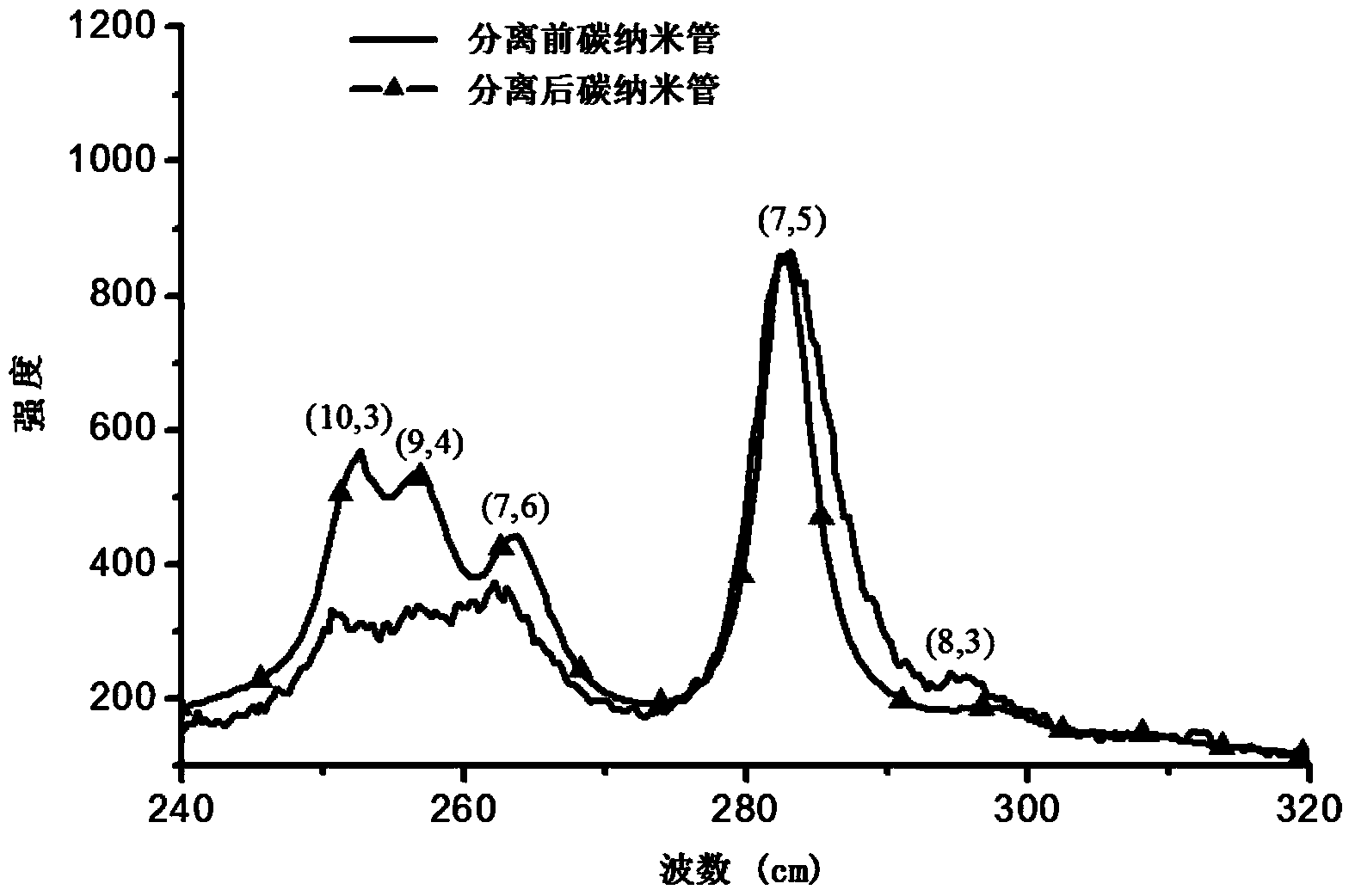
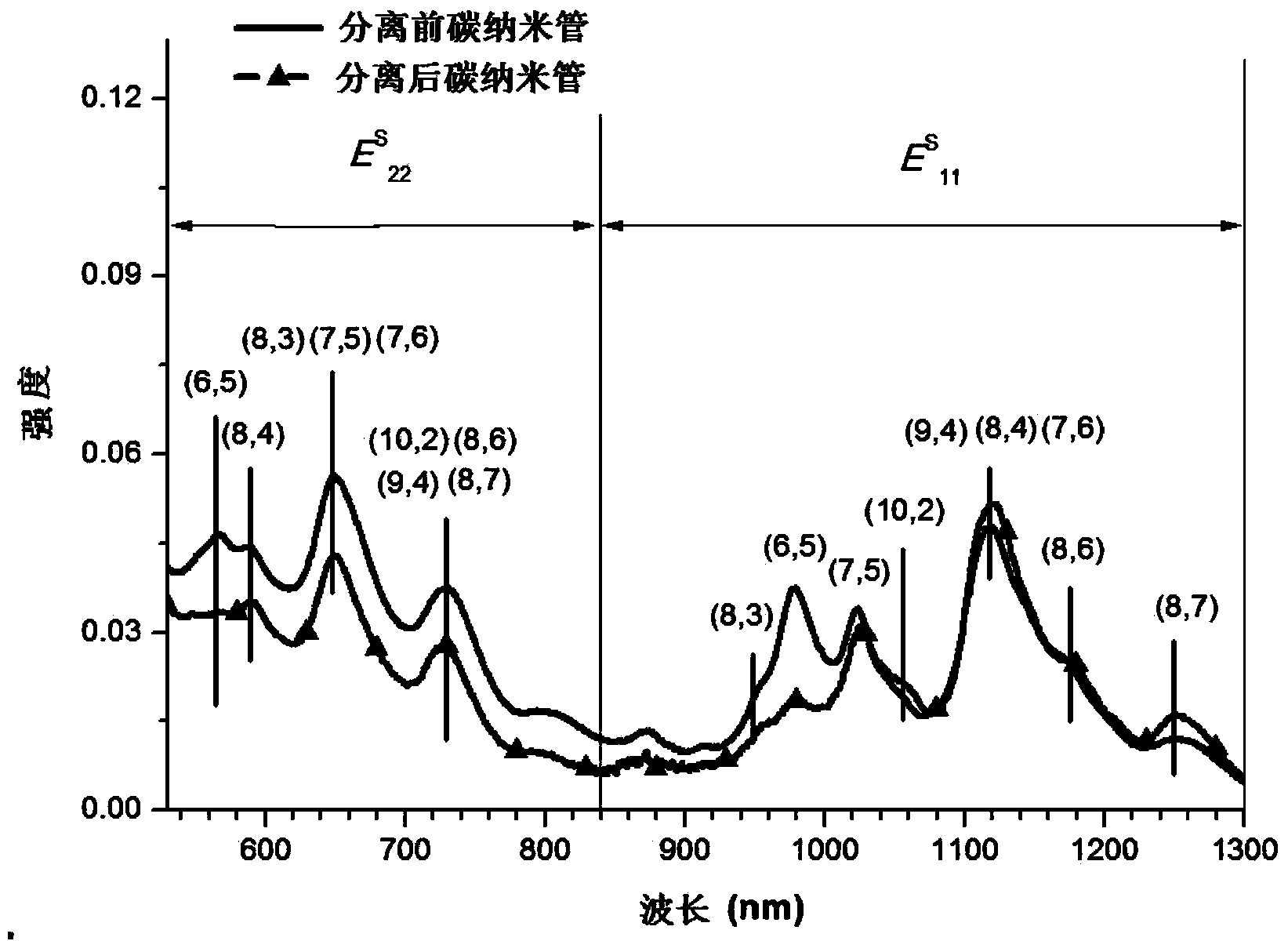
Aminocarbazole bridged molecular tweezer, and preparation method and applications thereof
A technology of aminocarbazole bridge and aminocarbazole dibromide is applied in the field of preparation of aminocarbazole bridged molecular tweezers, which can solve the problems of poor stability and poor molecular solubility of carbon nanotube composites, and achieve cost Inexpensive, good alcohol solubility, strong practical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of 3,6-dibromo-9-amino substituted carbazole
[0036] Take the preparation of 3,6-dibromo-9-(N,N-dimethylaminopropyl)carbazole as an example to illustrate.
[0037]
[0038] Under argon protection, add 100mL DMSO, 3,6-dibromocarbazole (6.5g, 20mmol), and 120mg tetrabutylammonium bromide to a 250ml flask respectively, stir well, add 12mL of 50% NaOH aqueous solution dropwise, continue After reacting for 30 minutes, 20 mL of an aqueous solution containing 6.3 g (40 mmol) of N,N-dimethylchlorpropylamine hydrochloride that had been neutralized with NaOH was added dropwise. After reacting for 6 hours, add 100 mL of water to the reaction system to dissolve the salt generated in the reaction, extract the reaction solution three times with 300 mL of ether, wash the organic phase with saturated brine three times, dry it with anhydrous sodium sulfate, and evaporate it under reduced pressure. The solvent was removed, and the crude product was recrystallized in a mi...
Embodiment 2
[0041] Preparation of 1-pinacol borate pyrene
[0042]
[0043]1-Bromopyrene (2.81g, 10mmol) was dissolved in dried tetrahydrofuran (THF, 100mL), and n-butyllithium (2.5M, 4.8mL, 12mmol) was added dropwise at -78°C. After reacting for 1 h, add 2-isopropyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3.7 mL, 18 mmol), then gradually warm to room temperature, and react for 24 h . The reaction mixture was poured into water and extracted with ether. The organic layer was washed with brine and dried over anhydrous calcium chloride. After the solvent was removed under reduced pressure, it was separated with a silica gel column, and the eluent was a mixed solution of petroleum ether and dichloromethane to obtain 2.33 g of white crystals with a yield of 71%. H NMR analysis results, 1 H NMR (300MHz, CDCl 3 ): 1.47 (12H, s, -CH 3 ), 7.99 (1H, t, PyrH), 8.06 (1H, d, PyrH), 8.16 (5H, m, PyrH), 8.53 (1H, d, PyrH), 8.99 (1H, d, PyrH). NMR tests showed that it was the target product. ...
Embodiment 3
[0045] Preparation of 2-pinacol borate pyrene:
[0046]
[0047] Add the catalyst [Ir(COD)Cl] in a 100mL three-neck flask under the protection of argon 2 (80mg), the ligand dtbpy (140mg) and a small amount of diboronic acid pinacol ester and 10mL n-hexane were dissolved, stirred vigorously for about 5min until the solution turned blue-purple, then added 4.04g (20mmol) pyrene and 5.58g (22mmol ) pinacol diboronate and 30 mL of n-hexane. After stirring and reacting in an oil bath at 80° C. for 24 h, the reaction mixture was passed through a short silica gel column to filter out the residual metal iridium catalyst. After the solvent was removed by rotary evaporation, 3.4 g of white solid was obtained by column separation, with a yield of 51.8%. H NMR analysis results, 1 H NMR (300MHz, CDCl 3 ): 1.46 (12H, s, -CH 3 ), 7.01 (1H, t, PyrH), 8.06 (4H, dd, PyrH), 8.20 (2H, d, PyrH), 8.59 (2H, s, PyrH). NMR tests showed that it was the target product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com