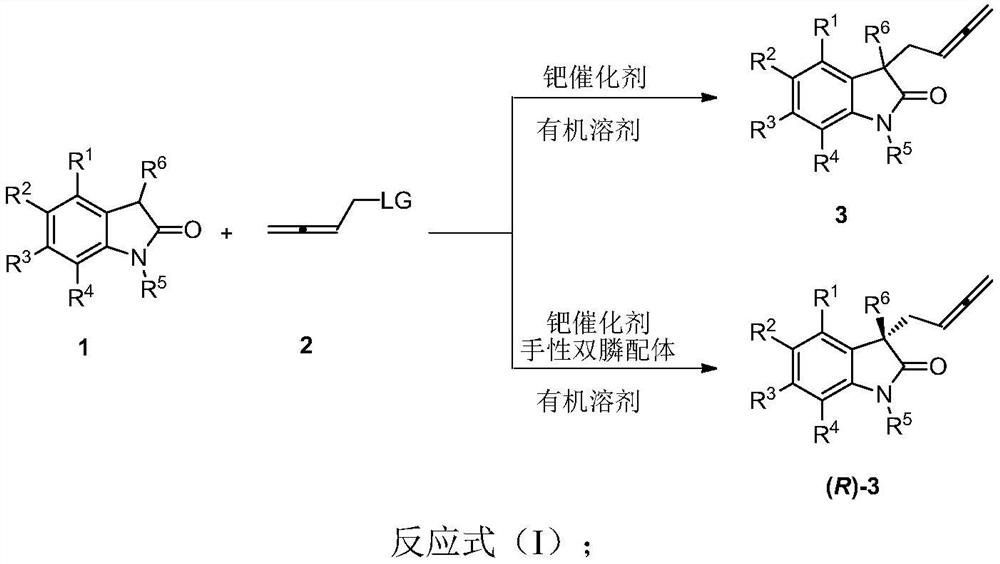
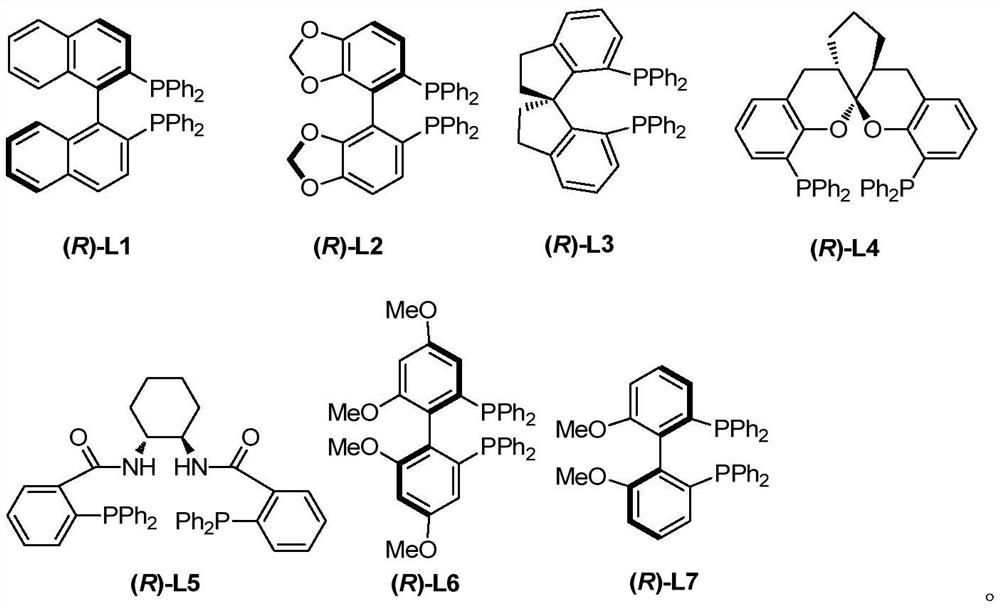
Racemic and chiral 3-(2, 3-butadienyl)oxoindolone compound, and preparation method and application thereof
A technology of indolinone and butadiene, which is applied in the field of transformation and antiviral application, can solve the problems that have not been studied, and achieve the effect of broad-spectrum antiviral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101]
[0102] Table 1
[0103]
[0104]
[0105] Operation steps: After the Schlenk reaction tube is dried by a heat gun, connect the argon gas cylinder to cool down to room temperature, pump and change the gas 3 times, add [Pd] (0.01mmol), 1a (0.24mmol), 2a in sequence under the protection of argon (0.2mmol), organic solvent (1.0mL), put into the preset 25 ℃ cold bath, stir for 12 hours; put the Schlenk reaction tube out of the cold bath, and use a silica gel short column (height: 3cm, diameter: 3.5cm) for the reaction solution ) filter, and rinse with 50mL of ether, concentrate, add 17.5 μL of dibromomethane, 0.8mL of deuterated chloroform, and analyze by proton nuclear magnetic spectrum, get racemic 3-(2,3-butadienyl) oxidolinone Compound 3aa NMR yield.
Embodiment 2
[0107]
[0108] After the Schlenk reaction tube was dried with a heat gun, it was connected to an argon gas cylinder and pumped to cool to room temperature, and the gas was exchanged for 3 times, and Pd(PPh 3 ) 4 (57.5mg, 0.05mmol), 1a (266.5mg, 1.2mmol), and 2a (203.8mg, 1.0mmol) / MeCN (5.0mL), the Schlenk reaction tube was placed in a preset 25 ° C oil bath, stirred for 5.5 hour (detected by thin-layer chromatography (TLC)), the Schlenk reaction tube was taken out of the oil bath and returned to room temperature, the reaction solution was filtered with a silica gel short column (height: 3cm, diameter: 3.5cm), and rinsed with 60mL of ether , concentrated, flash column chromatography [eluent: petroleum ether / ethyl acetate=15 / 1] to obtain racemic 3-(2,3-butadienyl) oxindolinone compound 3aa (246.7mg, 90%), oily liquid, 1 H NMR (400MHz, CDCl 3 )δ=7.37(d, J=7.6Hz, 2H, ArH), 7.36-7.18(m, 5H, ArH), 7.10(t, J=7.4Hz, 1H, ArH), 6.87(d, J=7.6Hz , 1H, ArH), 4.80-4.70 (quintet, J =...
Embodiment 3
[0110]
[0111] Operation is with embodiment 2. Pd(PPh 3 ) 4 (57.5mg, 0.05mmol), 1b (372.0mg, 1.2mmol), 2a (205.0mg, 1.0mmol), MeCN (5.0mL) reacted for 10.7 hours to give 3ba (318.1mg, 88%), an oily liquid, [first Sub-column chromatography, eluent: sherwood oil / ethyl acetate=30 / 1, collect all products The second column chromatography, eluent: sherwood oil / ether / dichloromethane=20 / 1 / 1] , 1 H NMR (400MHz, CDCl 3 )δ=7.94(d, J=8.0Hz, 1H, ArH), 7.40-7.15(m, 8H, ArH), 4.82-4.73(quintet, J=7.4Hz, 1H,=CH), 4.58-4.50(m ,1H, one protonof=CH), 4.50-4.40(m, 1H, one proton of=CH), 3.14-3.05(m, 1H, one proton of CH 2 ),3.00-2.88(m,1H,one proton of CH 2 ),1.61(s,9H,CH 3 ×3); 13 C NMR (100MHz, CDCl 3 )δ=210.2, 176.1, 149.3, 140.1, 139.1, 130.2, 128.61, 128.56, 127.7, 127.3, 125.2, 124.3, 115.2, 84.3, 84.1, 74.7, 56.8, 37.7, 28.1; -1):2999,2981,2930,2907,1945,1768,1724,1605,1477,1461,1418,1392,1369,1342,1284,1249,1214,1144,1101,1076,1045,1033,1000; MS( 70eV,EI)m / z(%):361[M + ,2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com