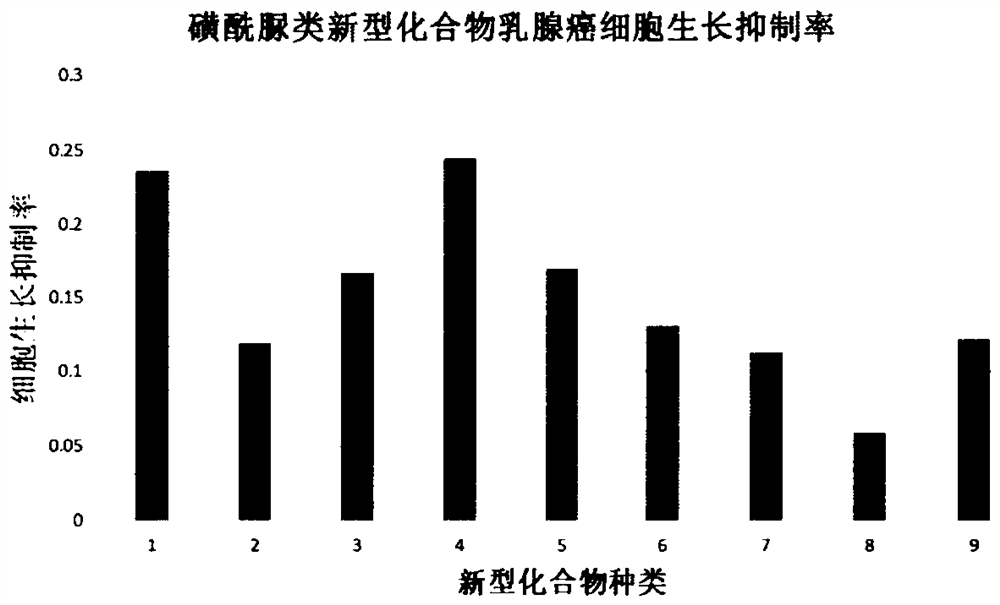
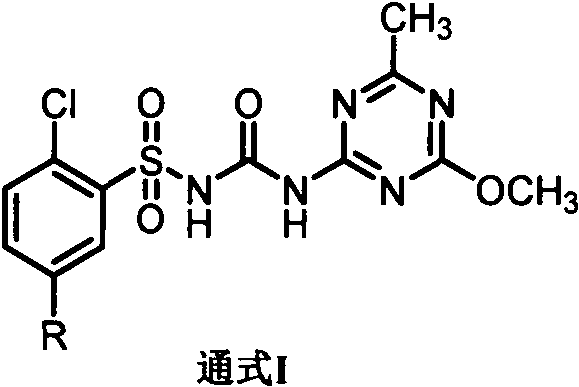
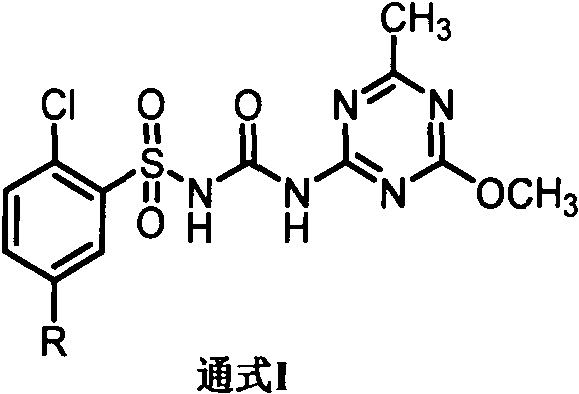
Application of chlorsulfuron benzene ring 5-substituted derivative in preparation of antitumor drugs
A technology of chlorsulfuron benzoyl ring and anti-tumor drug, which can be applied in the direction of anti-tumor drugs, drug combinations, active ingredients of heterocyclic compounds, etc., and can solve the problems of little research on anti-tumor compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] experimental method:
[0016] Compound configuration
[0017] (1) Weigh an appropriate amount of the new compound into a 1.5mL enzyme-free sterile EP tube, and mark the name of the compound on the side wall of the EP tube. According to the relative molecular mass of the compound, calculate the volume of DMSO added to make it The final concentration was 10 mM.
[0018] (2) Use a pipette gun to add the corresponding volume of DMSO in the ultra-clean bench of the cell room, and mix evenly by pipetting, so that the compound can be completely dissolved.
[0019] (3) Heat the 1.5mL EP tube in a 95°C metal bath for 10 minutes, then divide the compound solution into small tubes for later use.
[0020] (4) Store the compound solution in a refrigerator-20.
[0021] Preparation of MTT solution
[0022] (1) Take out a 50mL centrifuge tube in the ultra-clean bench, and add 20mL of sterilized PBS into it.
[0023] (2) Open the 100mg package of MTT, suck 1mL of PBS from the 50mL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com