Indole guanidine compound as well as preparation method and application thereof
A compound, the technology of indoguanidine, applied in the field of preparation of indoguanidine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
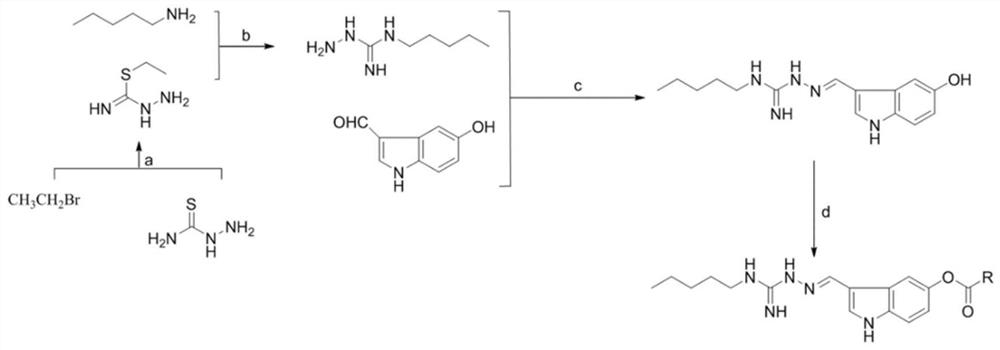
[0035] see figure 1 , the preparation method of the indoguanidine compound containing fatty acid ester of above-mentioned structure, comprises the following steps:
[0036] 1) thiosemicarbazide reacts with ethyl bromide to prepare thiobarbituric acid ethyl hydrazide;
[0037] 2) N-valeryl hydrazide carboxyxime amide is prepared by reacting ethyl thiobarbituryl hydrazide with pentylamine;
[0038] 3) Preparation of (E)-2-((5-hydroxy-1H-indol-3-yl)methylene)-N - Amylhydrazine-1-carboxamide;
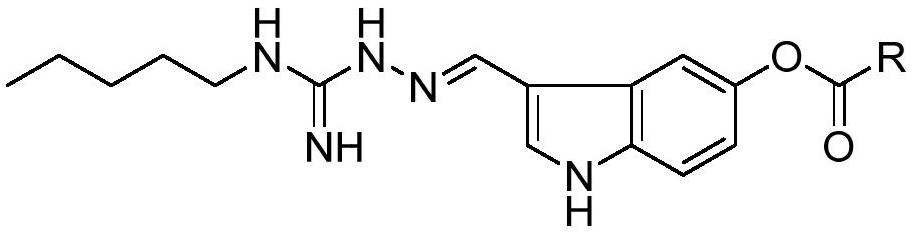
[0039] 4) Condensation of (E)-2-((5-hydroxy-1H-indol-3-yl)methylene)-N-pentylhydrazine-1-carboxamide with fatty acid to generate indoguanidine containing fatty acid ester class of compounds.
[0040] The specific process of the step 1) is: add thiosemicarbazide and absolute ethanol into an eggplant-shaped bottle, heat up the oil bath to 55-65°C, then add bromoethane to react for 5-7h, after the reaction is completed, the reaction system Cool to room temperature, wait for a large amount...
Embodiment 1
[0046] A kind of indoguanidine compound, R is , the preparation method is as follows:
[0047] 1) Synthesis of ethyl thiobarbiturohydrazide: 2g of thiosemicarbazide (21.95mmol) and 40mL of absolute ethanol were added to a 100mL eggplant-shaped bottle, the oil bath was heated to 60°C, and 3.588g of bromoethane ( 32.925mmol) for 6h. After the reaction was completed, the reaction system was cooled to room temperature, and a large amount of white solid was precipitated in the bottle, filtered, and the filter cake was washed with anhydrous ether and dried to constant weight to obtain white crystal ethyl thiobarbiturohydrazide with a yield of 97%. EI-MS(m / z):120[M] - .
[0048] 2) Synthesis of N-valeryl hydrazide carboximide: add 1 g of ethyl thiobarbituryl hydrazide (8.390 mmol) and 20 mL of methanol into a 100 mL eggplant-shaped bottle, heat the oil bath to 65°C, and add 0.878 g of n-pentyl hydrazide Amine (10.068mmol), reflux for 5h. After the reaction was completed, the so...
Embodiment 2
[0052] A kind of indoguanidine compound, R is , the preparation method is as follows:
[0053] 1) Step 1) to step 4) are the same as in Example 1, except that 5-hexynoic acid is replaced by 6-heptynoic acid to obtain white solid compound 2 ((E)-3-((2-(N -Verylcarbamoyl)hydrazone)methyl)-1H-indol-5-ylhept-6-ynoate, yield 40%; EI-MS(m / z):396[M] - ;394[M] + . Purity: 97.560%. 1 H NMR(400MHz,DMSO)δ11.85(s,1H),11.35(s,1H),8.38(s,1H),7.95(d,J=3.7Hz,1H),7.77(s,1H),7.67 (s,1H),7.45(d,J=8.7Hz,1H),6.94(dd,J=8.7,2.2Hz,1H),3.28(d,J=4.9Hz,2H),2.80(t,J= 2.6Hz, 1H), 2.61(t, J=7.4Hz, 2H), 2.50(s, 1H), 2.23(td, J=6.9, 2.6Hz, 2H), 1.76(dt, J=15.2, 7.4Hz, 2H),1.61–1.57(m,2H),1.55(d,J=2.8Hz,2H),1.36–1.29(m,4H),0.89(t,J=6.5Hz,3H).
[0054] The synthesis steps of compound 3 are the same as those in Example 1, except that 5-hexynoic acid is replaced by levulinic acid.
[0055] Compound 3, white solid, yield 42%. EI-MS(m / z):386[M] - ;384[M] + . Purity: 95.996%; 1 HNMR(400MHz,DMSO)δ11.85...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com