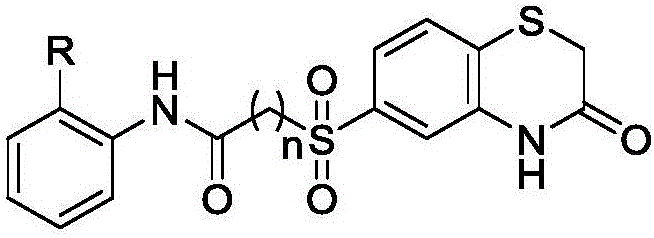
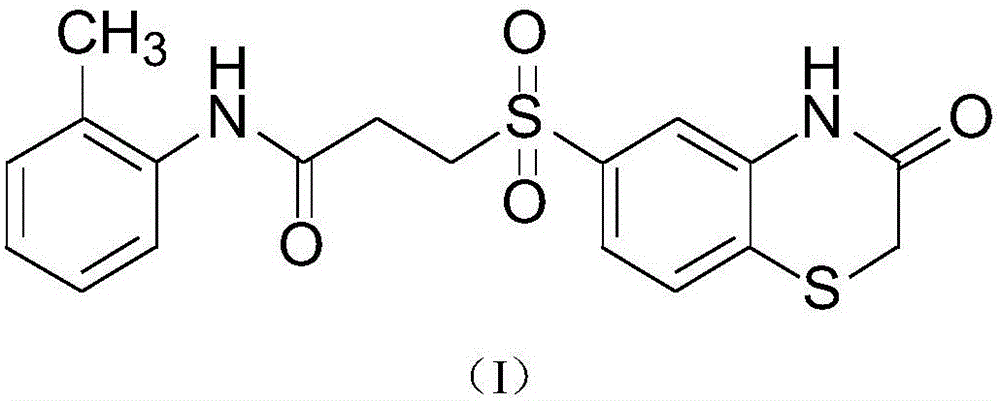
Antitumor application of small-molecule organic compound
A compound and anti-tumor drug technology, applied in the field of medicinal chemistry, can solve problems such as few structural types, low inhibition efficiency, and unclear mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Antitumor activity test of compound (I) (FN-01)
[0016] Compound FN-01 of the present invention has been carried out IDO1 inhibitory experiment, following is the pharmacological test and result of compound of the present invention:
[0017] IDO enzyme can catalyze the pyrrole epoxidation cleavage on tryptophan to produce N'-formylkynurenine. At room temperature, mix 40nM IDO enzyme and 900uM L-tryptophan, add reaction buffer (20mMascorbate, 3.5uMmethyleneblueand0.2mg / mLcatalasein50mMpotassiumphosphatebufferpH6.5), react at room temperature for three hours, and then The ultraviolet measurement is carried out on the instrument, and the detection wavelength is 321nm. The instrument reading is converted into: % activity = [(A-Ab) / (At-Ab)] × 100, and then use PrismGraphPadsofeware software to fit and calculate IC 50 value.
[0018] The test results are shown in Table 1:
[0019] Table 1. FN-01 inhibition test on IDO1
[0020] sample number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com