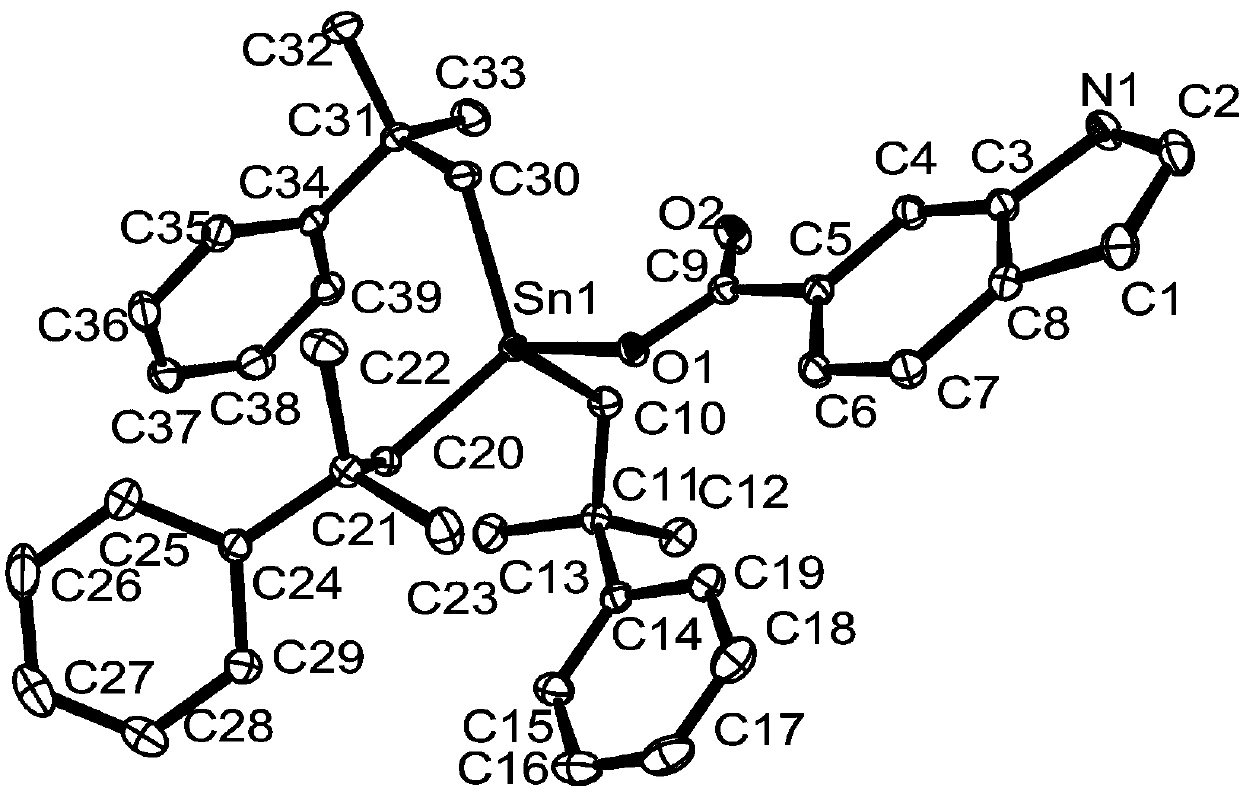
Preparation method and application of tri(2-methyl-2-phenylpropyl)tin indole-6-carboxylate complex
A technology of phenylpropyl and complexes, which is applied in the directions of tin organic compounds, chemical instruments and methods, compounds of elements of Group 4/14 of the periodic table, etc., can solve problems such as application limitation and strong toxicity, and achieve a simple preparation method. , low cost, good anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of three (2-methyl-2-phenylpropyl) tin indole-6-carboxylate complexes:
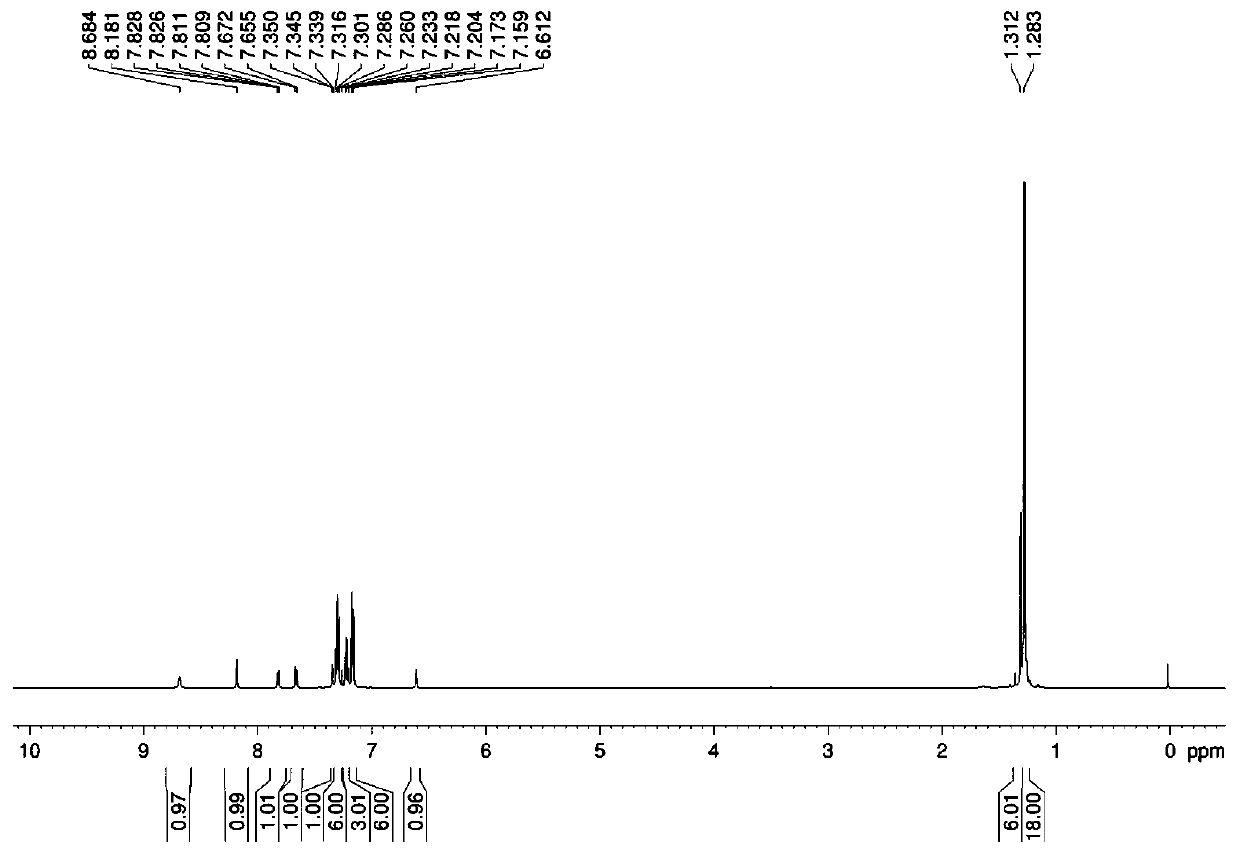
[0034] Add bis[tris(2-methyl-2-phenylpropyl)]tin oxide 1.0537 g (1.0 mmol), indole-6-carboxylic acid 0.3234 g (2.0 mmol), solvent-free With 10 mL of water and methanol, the microwave reaction was carried out under an air atmosphere with a radiation power of 800 W and a temperature of 100 °C for 60 min. After the reaction, cool naturally, filter, and the solvent volatilizes naturally at room temperature to crystallize to obtain white crystals, namely tris(2-methyl-2-phenylpropyl)tinindole-6-carboxylate complex. Yield: 63%, melting point: 141-143°C.
[0035] Elemental analysis (C 39 h 45 NO 2 Sn): theoretical values: C, 69.04; H, 6.69; N, 2.06. Found: C, 69.07; H, 6.62; N, 2.01.
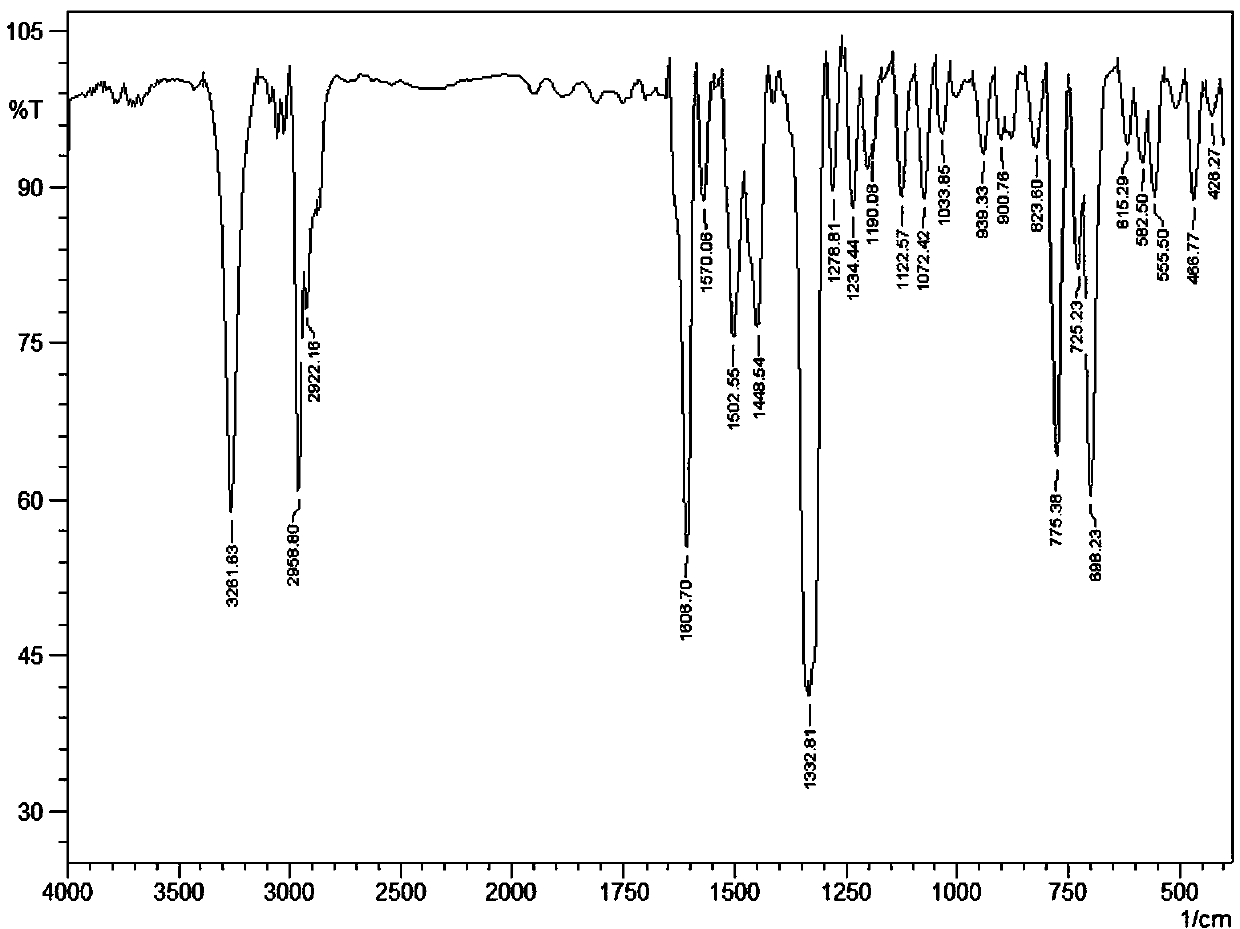
[0036] IR(KBr, v / cm -1 ): 3261.63 (s), 2958.80 (s), 2922.16 (m), 1606.70 (s), 1570.06 (w), 1502.55 (m), 1448.54 (m), 1332.81 (s), 1278.81 (w), 1234.44 (w ), 1190.08 (w), 1122.57 (w), 1072.42 (w), 1033.85...
Embodiment 2
[0042] Preparation of three (2-methyl-2-phenylpropyl) tin indole-6-carboxylate complexes:
[0043] Add bis[tris(2-methyl-2-phenylpropyl)]tin oxide 1.0541 g (1.0 mmol), indole-6-carboxylic acid 0.3557 g (2.1 mmol), solvent-free With 15 mL of water and methanol, the microwave reaction was carried out under an air atmosphere with a radiation power of 800 W and a temperature of 100 °C for 60 min. After the reaction, cool naturally, filter, and the solvent volatilizes naturally at room temperature to crystallize to obtain white crystals, namely tris(2-methyl-2-phenylpropyl)tinindole-6-carboxylate complex. Yield: 64%, melting point: 141-143°C.
[0044] Elemental analysis (C 39 h 45 NO 2 Sn): theoretical values: C, 69.04; H, 6.69; N, 2.06. Found: C, 69.07; H, 6.62; N, 2.01.
[0045] IR(KBr, v / cm -1 ): 3261.63 (s), 2958.80 (s), 2922.16 (m), 1606.70 (s), 1570.06 (w), 1502.55 (m), 1448.54 (m), 1332.81 (s), 1278.81 (w), 1234.44 (w ), 1190.08 (w), 1122.57 (w), 1072.42 (w), 1033.85...
Embodiment 3
[0051] Preparation of three (2-methyl-2-phenylpropyl) tin indole-6-carboxylate complexes:
[0052]Add bis[tris(2-methyl-2-phenylpropyl)]tin oxide 1.0534 g (1.0 mmol), indole-6-carboxylic acid 0.3230 g (2.0 mmol), solvent-free With 12 mL of water and methanol, the microwave reaction was carried out at a radiation power of 800 W and a temperature of 100 °C in an air atmosphere for 120 min. After the reaction, cool naturally, filter, and the solvent volatilizes naturally at room temperature to crystallize to obtain white crystals, namely tris(2-methyl-2-phenylpropyl) tin indole-6-carboxylate complex. Yield: 65%, melting point: 141-143°C.
[0053] Elemental analysis (C 39 h 45 NO 2 Sn): theoretical values: C, 69.04; H, 6.69; N, 2.06. Found: C, 69.07; H, 6.62; N, 2.01.
[0054] IR(KBr, v / cm -1 ): 3261.63 (s), 2958.80 (s), 2922.16 (m), 1606.70 (s), 1570.06 (w), 1502.55 (m), 1448.54 (m), 1332.81 (s), 1278.81 (w), 1234.44 (w ), 1190.08 (w), 1122.57 (w), 1072.42 (w), 1033.85 (w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com