Preparation method and application of tricyclohexyltin indole-3-carboxylate complex
A technology of tricyclohexyl and complexes, applied in tin organic compounds, pharmaceutical formulations, organic chemical methods, etc., can solve problems such as application limitation, strong toxicity, etc., and achieve the effects of simple preparation method, low cost, and high anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
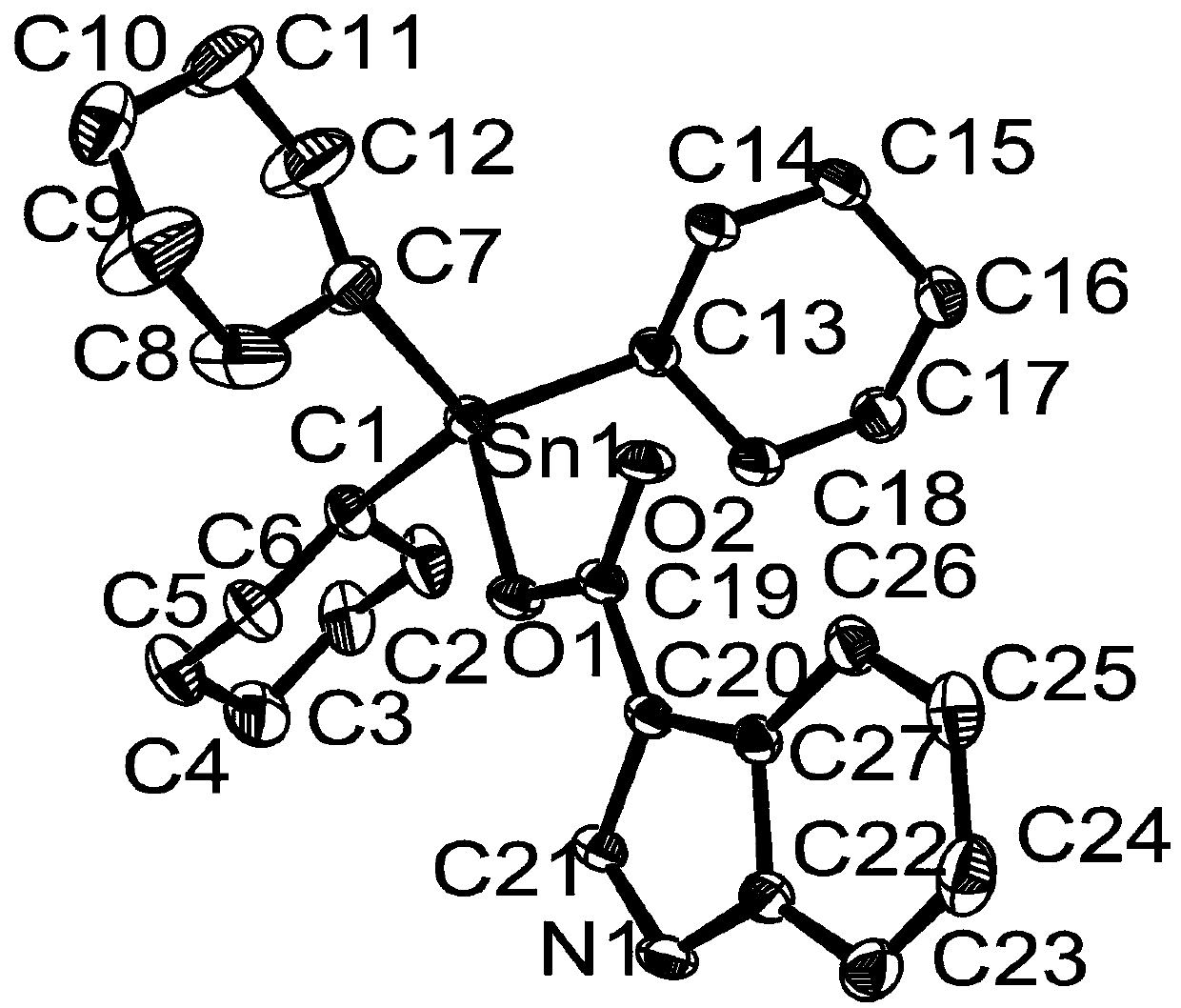
[0033] Preparation of tricyclohexyltin indole-3-carboxylate complex:
[0034] Add 0.3857 g (1 mmol) of tricyclohexyltin hydroxide, 0.1617 g (1 mmol) of indole-3-carboxylic acid, and 10 mL of anhydrous methanol into the microwave reaction tank in sequence. Microwave reaction was performed at 800 W and 100 °C for 60 min. After the reaction, cool naturally, filter, and the solvent volatilizes and crystallizes at room temperature to obtain white crystals, which are tricyclohexyl tin indole-3-carboxylate complexes. Yield: 66%, melting point: 222-225°C.
[0035] Elemental analysis (C27 h 39 NO 2 Sn): theoretical values: C, 61.38; H, 7.44; N, 2.65. Found: C, 61.38; H, 7.43; N, 2.69.
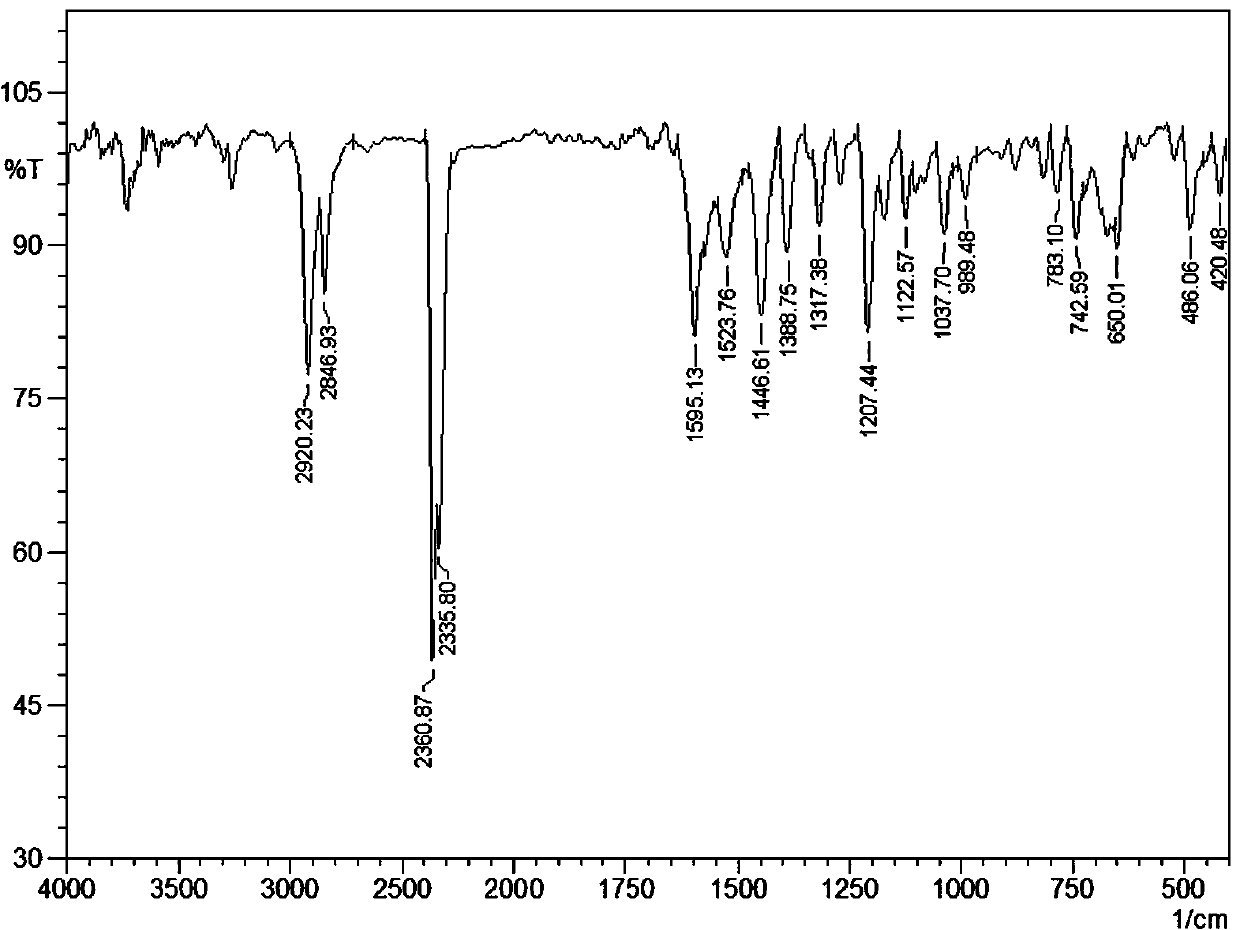
[0036] IR(KBr, v / cm -1 ): 2920.23 (m), 2846.93 (m), 2360.87 (s), 2335.80 (s), 1595.13 (m), 1523.76 (m), 1446.61 (m), 1388.75 (m), 1317.38 (w), 1207.44 (m ), 1122.67 (w), 1037.70 (m), 989.48 (w), 783.10 (w), 742.59 (m), 650.01 (m), 486.06 (m), 420.48 (w).
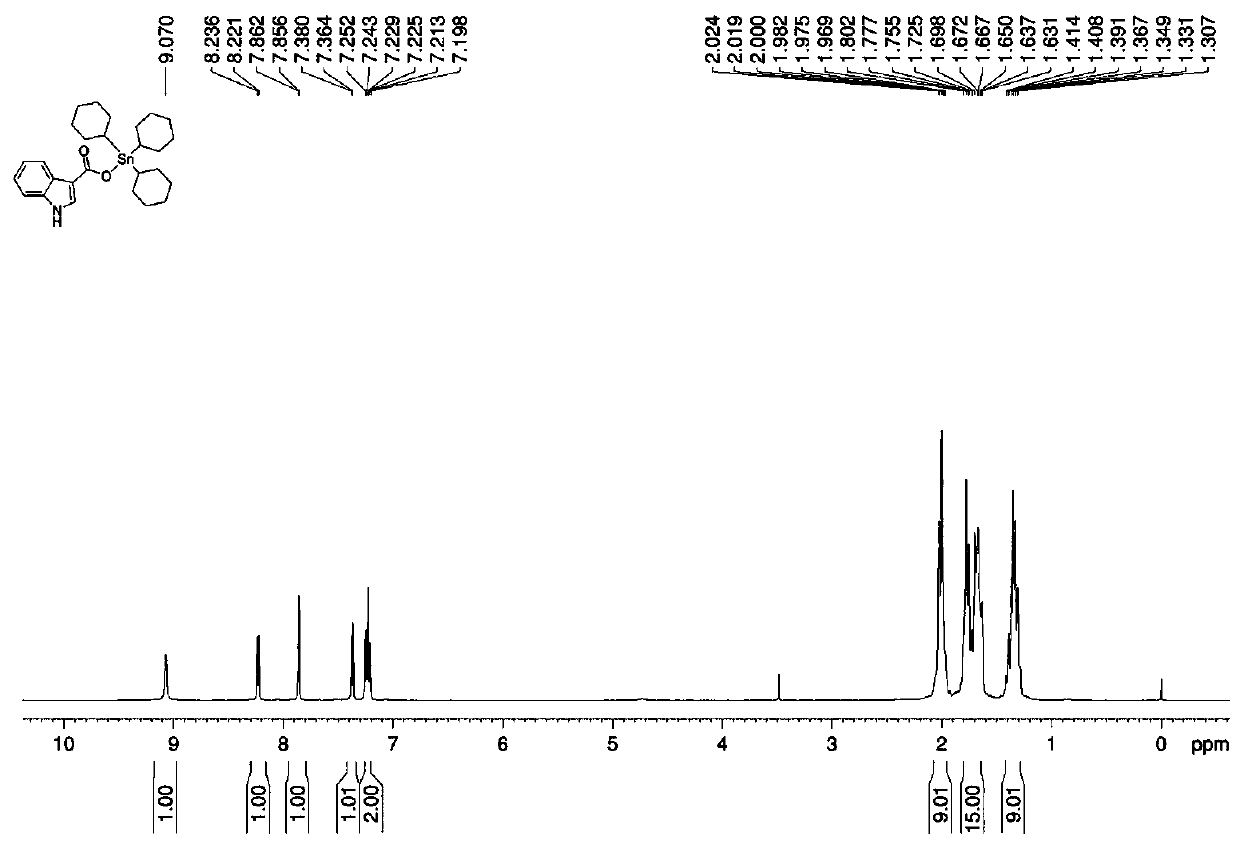
[0037] 1 H NMR (CDCl 3 , 500 MHz) δ ...
Embodiment 2
[0042] Preparation of tricyclohexyltin indole-3-carboxylate complex:
[0043] Add 0.3846 g (1 mmol) of tricyclohexyltin hydroxide, 0.1693 g (1.05 mmol) of indole-3-carboxylic acid, and 15 mL of anhydrous methanol into the microwave reaction tank in sequence. Microwave reaction was performed at 800 W at 100 °C for 60 min. After the reaction, cool naturally, filter, and the solvent volatilizes and crystallizes at room temperature to obtain white crystals, which are tricyclohexyl tin indole-3-carboxylate complexes. Yield: 67%, melting point: 222-225°C.
[0044] Elemental analysis (C 27 h 39 NO 2 Sn): theoretical values: C, 61.38; H, 7.44; N, 2.65. Found: C, 61.38; H, 7.43; N, 2.69.
[0045] IR(KBr, v / cm -1 ): 2920.23 (m), 2846.93 (m), 2360.87 (s), 2335.80 (s), 1595.13 (m), 1523.76 (m), 1446.61 (m), 1388.75 (m), 1317.38 (w), 1207.44 (m ), 1122.67 (w), 1037.70 (m), 989.48 (w), 783.10 (w), 742.59 (m), 650.01 (m), 486.06 (m), 420.48 (w).
[0046] 1 H NMR (CDCl 3 , 500 MHz)...
Embodiment 3
[0051] Preparation of tricyclohexyltin indole-3-carboxylate complex:
[0052] Add 0.3848 g (1 mmol) of tricyclohexyltin hydroxide, 0.1620 g (1 mmol) of indole-3-carboxylic acid, and 12 mL of anhydrous methanol into the microwave reaction tank in sequence. Microwave reaction was performed at 800 W and 100 °C for 120 min. After the reaction, cool naturally, filter, and the solvent volatilizes and crystallizes at room temperature to obtain white crystals, which are tricyclohexyl tin indole-3-carboxylate complexes. Yield: 68%, melting point: 222-225°C.
[0053] Elemental analysis (C 27 h 39 NO 2 Sn): theoretical values: C, 61.38; H, 7.44; N, 2.65. Found: C, 61.38; H, 7.43; N, 2.69.
[0054] IR(KBr, v / cm -1 ): 2920.23 (m), 2846.93 (m), 2360.87 (s), 2335.80 (s), 1595.13 (m), 1523.76 (m), 1446.61 (m), 1388.75 (m), 1317.38 (w), 1207.44 (m ), 1122.67 (w), 1037.70 (m), 989.48 (w), 783.10 (w), 742.59 (m), 650.01 (m), 486.06 (m), 420.48 (w).
[0055] 1 H NMR (CDCl 3 , 500 MHz) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com