Novel chlorine-containing carboxylic acid metal complex, and preparation method and application thereof
A technology of metal complexes and chlorocarboxylic acids, applied in the direction of 1/11 organic compounds without C-metal bonds, copper organic compounds, organic chemical methods, etc., can solve the problems of large toxic and side effects, hindered use, and strong drug resistance and other problems, to achieve the effect of low equipment requirements, simple preparation method, and induction of apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
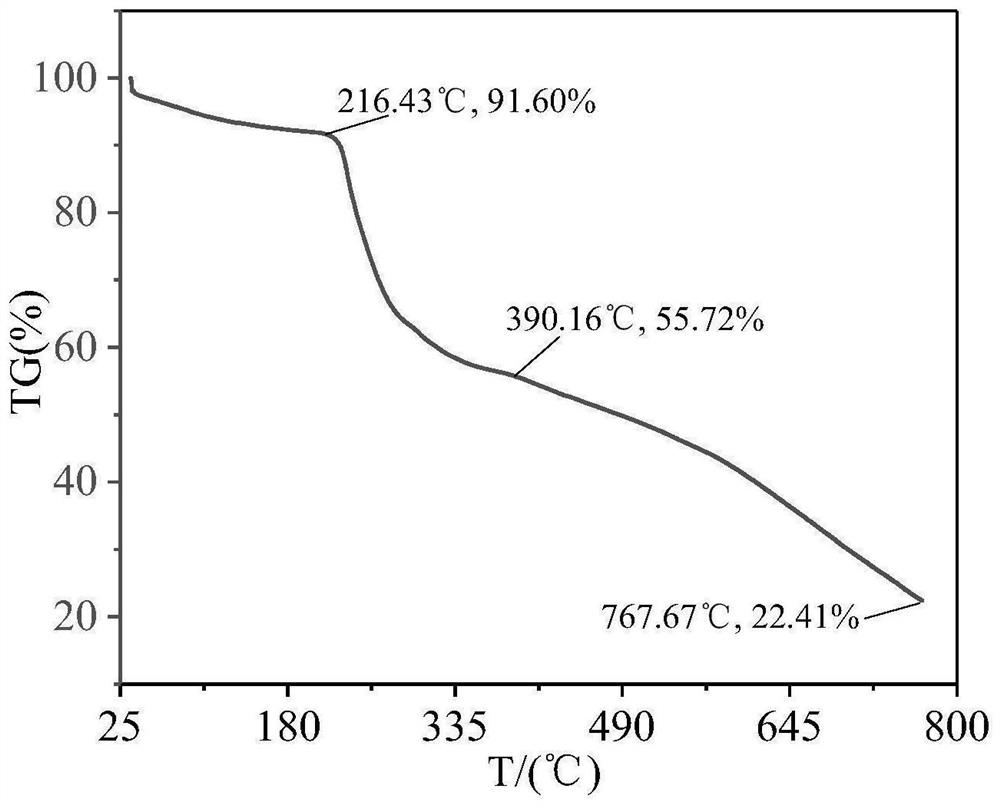
Image
Examples
preparation example Construction
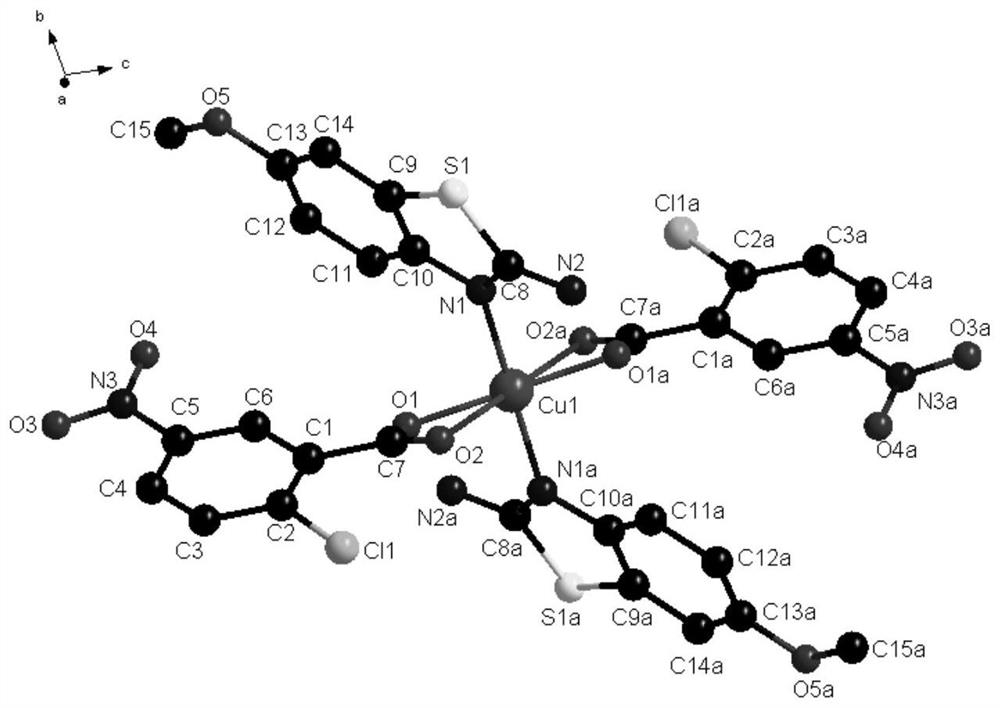
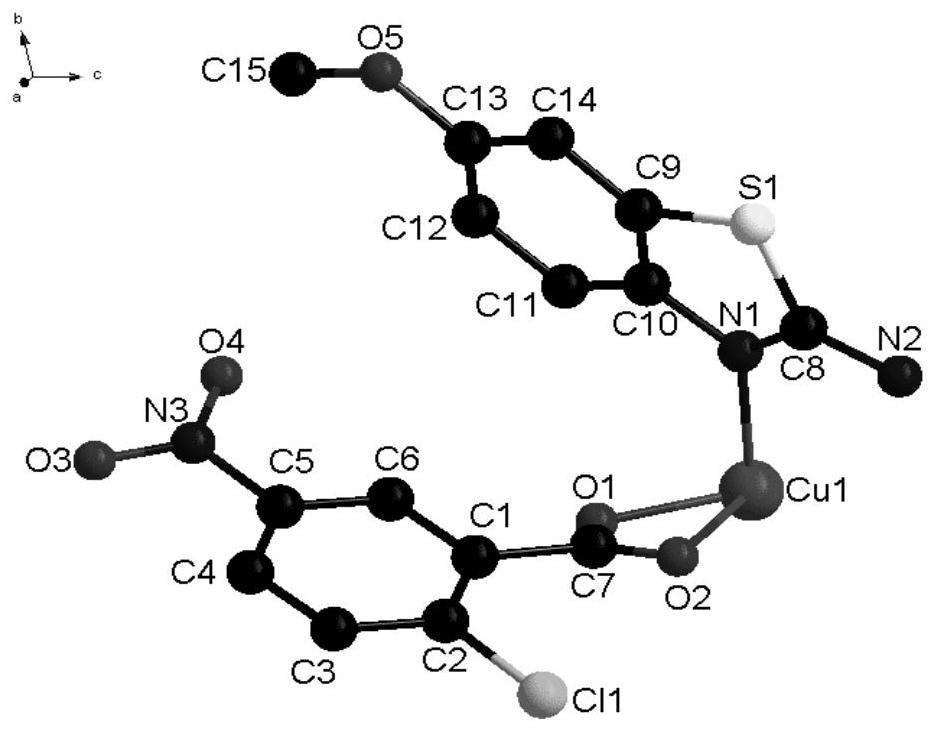
[0038] In some embodiments of the present invention, a method for preparing the above-mentioned novel chlorocarboxylic acid metal complexes is also provided, comprising the following steps: 2-chloro-5-nitrobenzoic acid, 2-amino-6-methoxybenzene And thiazole is dissolved in the solvent, stirred and dissolved to make a mixed solution; copper nitrate is dissolved in the solvent, stirred and dissolved to obtain a copper nitrate solution, and the copper nitrate solution is added dropwise to the mixed solution. After the drop is completed, adjust the pH at 5-7 In between, stir and react at room temperature for a period of time, seal the film and place it in a cool place, and after standing for 12-28d, brown blocky crystals are precipitated.
[0039] In order to fully react the ligand raw materials and obtain the target complex, the molar ratio of the 2-chloro-5-nitrobenzoic acid, 2-amino-6-methoxybenzothiazole and copper nitrate is 9:3:1.5 -2.
[0040] In order to make the raw mate...
Embodiment 1
[0046]Synthesis of the complex: Dissolve 2-chloro-5-nitrobenzoic acid (0.9mmol) and 2-amino-6-methoxybenzothiazole (0.3mmol) in 5ml of methanol, stir and dissolve to make a mixed solution; Dissolve copper nitrate (0.15mmol) in 10ml of methanol solvent, stir and dissolve to obtain a copper nitrate solution, add the copper nitrate solution dropwise to the mixed solution within 20min, the solution appears yellow-green, after the addition is complete, use 1.0mol L -1 The NaOH solution adjusted the pH to 5, stirred and reacted at room temperature for 20 minutes, sealed the film and placed it in a cool place, and after standing for 12 days, brown blocky crystals were precipitated.
Embodiment 2
[0048] Synthesis of the complex: Dissolve 2-chloro-5-nitrobenzoic acid (0.9mmol) and 2-amino-6-methoxybenzothiazole (0.3mmol) in 5ml of methanol, stir and dissolve to make a mixed solution; Dissolve copper nitrate (0.18mmol) in 10ml of methanol solvent, stir and dissolve to obtain a copper nitrate solution, add the copper nitrate solution dropwise to the mixed solution within 30min, the solution appears yellow-green, after the addition is complete, use 1.0mol·L -1 The NaOH solution adjusted the pH to 6, stirred and reacted at room temperature for 30 minutes, sealed the film and placed it in a cool place, and after standing for 20 days, tan massive crystals were precipitated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com