Organotin-tetraphenyl ethylene acylhydrazone compound with AIE characteristic as well as preparation method and application of organotin-tetraphenyl ethylene acylhydrazone compound
A technology of tetraphenylethylene acylhydrazone compound and tetraphenylethylene, applied in tin organic compounds, chemical instruments and methods, drug combinations, etc., can solve problems such as luminescence quenching, and achieve good anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
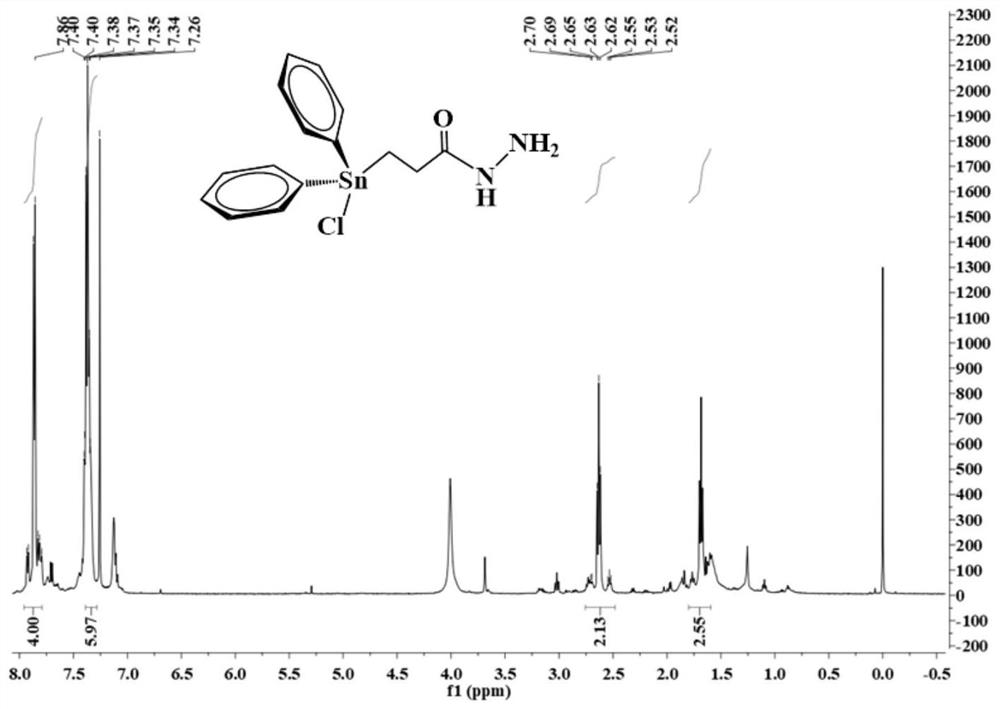
[0050] Under electromagnetic stirring, 0.44 g of triphenyltinhydrazine carbonyl compound (L1) was dissolved in 20 mL of chloroform, and then 0.16 g of chloroform solution of iodine chloride was slowly added dropwise to the above solution under an ice-water bath. Faded, the dropwise addition was completed, and the reaction was continued for 30 min to obtain a colorless solution. The solvent was evaporated under reduced pressure to obtain a colorless viscous substance, which solidified after being placed in a vacuum desiccator for two days. The colorless crystal compound (L2) 0.37 g was recrystallized with chloroform-petroleum ether in a volume ratio of 1:1, and the yield was 93.2 %. Characterization spectra such as figure 1 shown: 1 H NMR (500 MHz, CDCl 3 ) δ 7.98-7.74 (m, 4H), 7.45-7.25 (m, 6H), 2.75-2.42 (m, 2H), 1.79-1.57 (m, 2H).
Embodiment 2
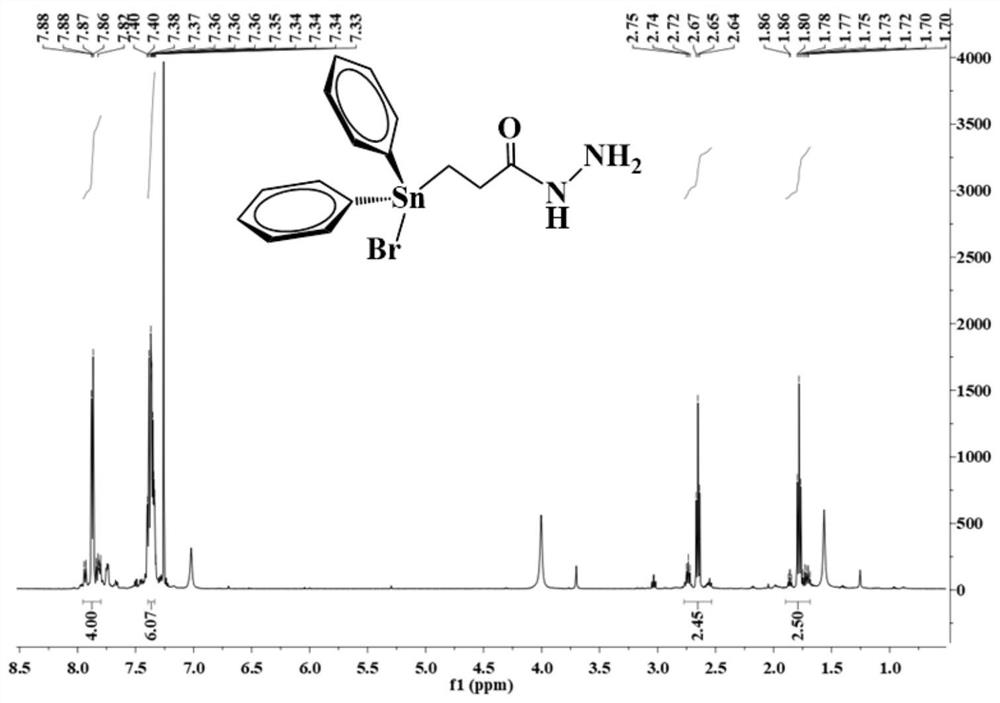
[0052] Under electromagnetic stirring, 0.44 g of triphenyltinhydrazine carbonyl compound (L1) was dissolved in 20 mL of chloroform, and then 0.16 g of the chloroform solution of elemental bromine was slowly added dropwise to the above solution under an ice-water bath, and the color faded immediately. , the dropwise addition was completed, and the reaction was continued for 30 min to obtain a colorless solution. The solvent was evaporated under reduced pressure to obtain a colorless viscous substance, which solidified after being placed in a vacuum desiccator for two days. The colorless crystal compound (L3) 0.41 g was recrystallized with a volume ratio of 1:1 chloroform-petroleum ether, and the yield was 93.2 %. Characterization spectra such as figure 2 shown: 1 H NMR (500 MHz, CDCl 3 ) δ 7.96-7.78 (m, 4H), 7.42-7.31 (m, 6H), 2.69 (dt, 3 J ( 119 Sn-H)=15.3, 7.3 Hz, 2H), 1.91-1.68 (m, 2H).
Embodiment 3
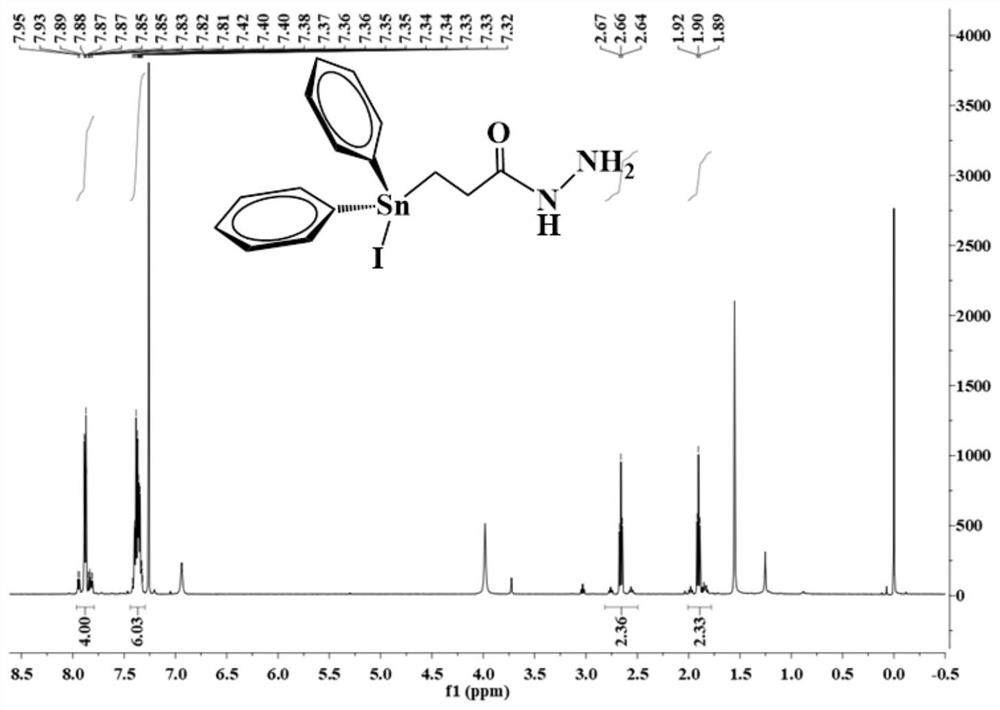
[0054] Under electromagnetic stirring, 0.44 g of triphenyltinhydrazine carbonyl compound (L1) was dissolved in 20 mL of chloroform, and then 0.25 g of the chloroform solution of elemental iodine was slowly added dropwise to the above solution under an ice-water bath, and the color faded immediately. , the dropwise addition was completed, and the reaction was continued for 30 min to obtain a colorless solution. The solvent was evaporated under reduced pressure to obtain a colorless viscous substance, which solidified after being placed in a vacuum desiccator for two days. The colorless crystal compound (L4) 0.42 g was recrystallized with chloroform-petroleum ether in a volume ratio of 1:1, and the yield was 85.7 g %. Characterization spectra such as image 3 shown: 1 H NMR (500 MHz, CDCl 3 ) δ 7.99-7.78 (m, 4H), 7.47-7.27 (m, 6H), 2.66 (t, 3 J ( 119 Sn-H)= 10.0 Hz, 2H), 1.90 (t, 3 J ( 119 Sn-H) =10.0 Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com