Synthesis and application of HDAC6 inhibitor based on hydroxamic acid
A technology of inhibitors and uses, applied in the field of medicinal chemistry, can solve the problems of lack of HDAC subtype selectivity, adverse reactions, etc., and achieve the effects of good anti-cancer activity, simple post-processing, and good economy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1 synthetic method:
[0054]
[0055] Preparation of intermediates
[0056] Intermediate: Preparation of (E)-2-(1-(2-phenylhydrazinoethylidene)aniline (3)
[0057] 2-Aminoethanone (0.5 mmol), phenylhydrazine (0.5 mmol) and p-toluenesulfonic acid (0.05 mmol) were refluxed in 2 ml of ethanol at 80 degrees for 8 hours. The reactant was dried under reduced pressure and washed with n-hexane to obtain the product (E)-2-(1-(2-phenylhydrazinoethylidene)aniline, yield: 82%.
[0058] Intermediate: Preparation of 2-(1H-indol-2-yl)aniline (4)
[0059] 1ml of Eaton's reagent was stirred and heated at 80 degrees in the flask, when the temperature was constant, reactant 3 (0.5mol) was added and stirred for 30min, cooled to room temperature, and the pH was adjusted to 7 with 0.2mol / L aqueous sodium hydroxide solution under ice bath conditions, After forming a precipitate, suction filtration and washing with deionized water for 3 times to obtain the product 2-(1H-indol-...
Embodiment 2
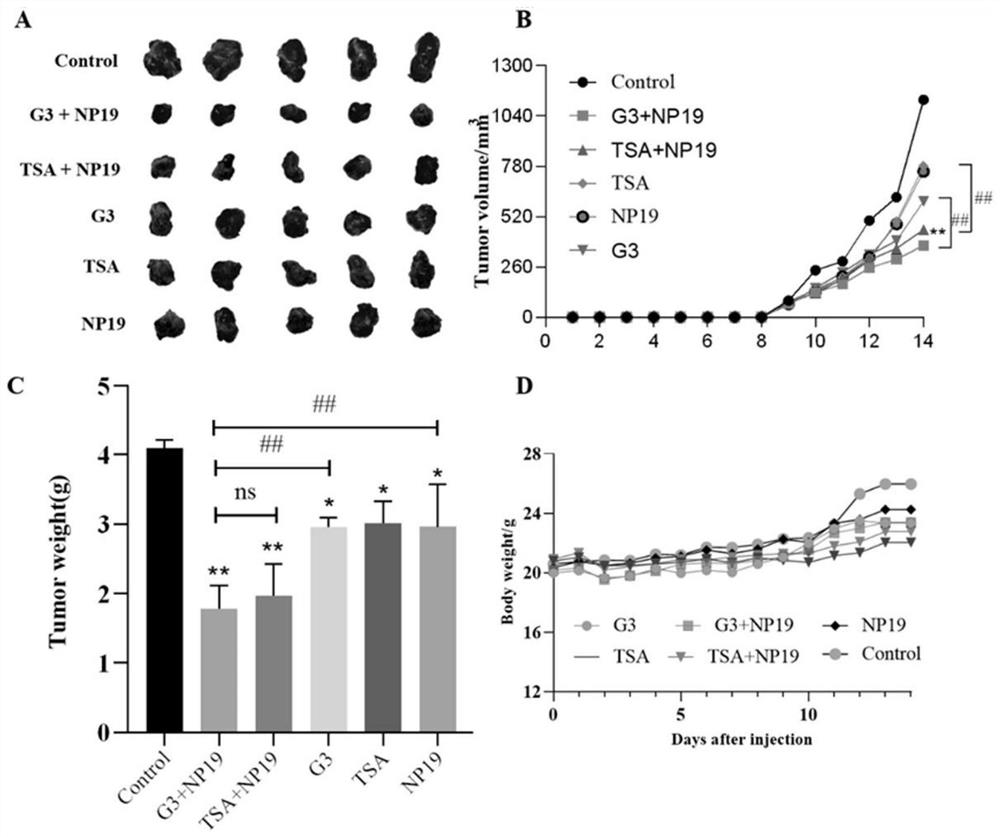
[0071] Example 2 In vitro antitumor activity study
[0072] The in vitro antitumor activity of the compounds of the present invention was demonstrated by the following method. These effects suggest that the compounds of the present invention are useful in the treatment of cancer. The specific test method is as follows:
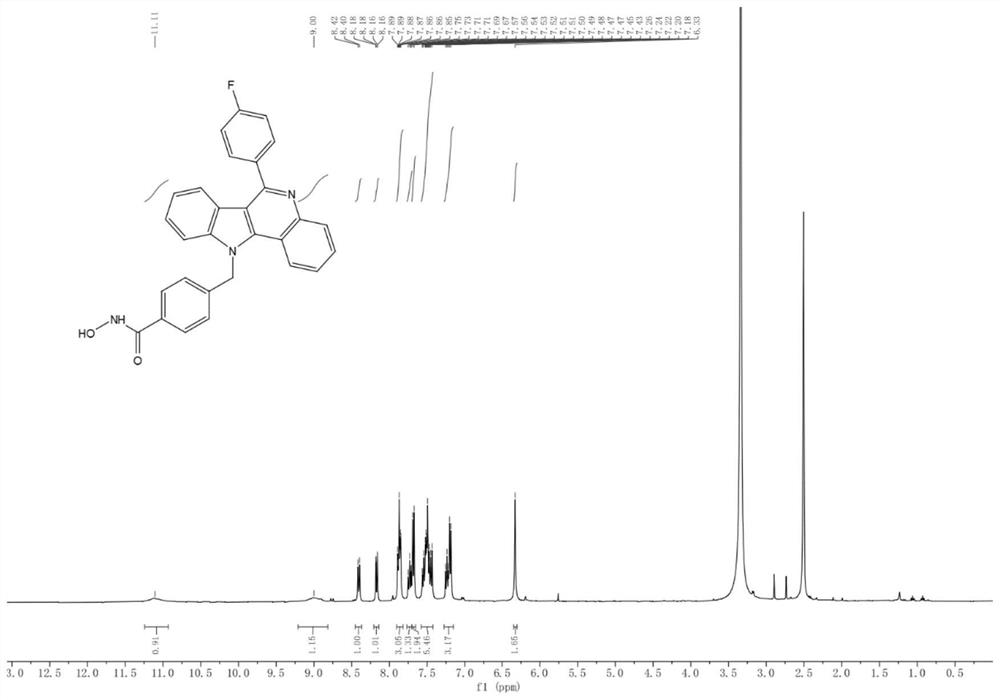
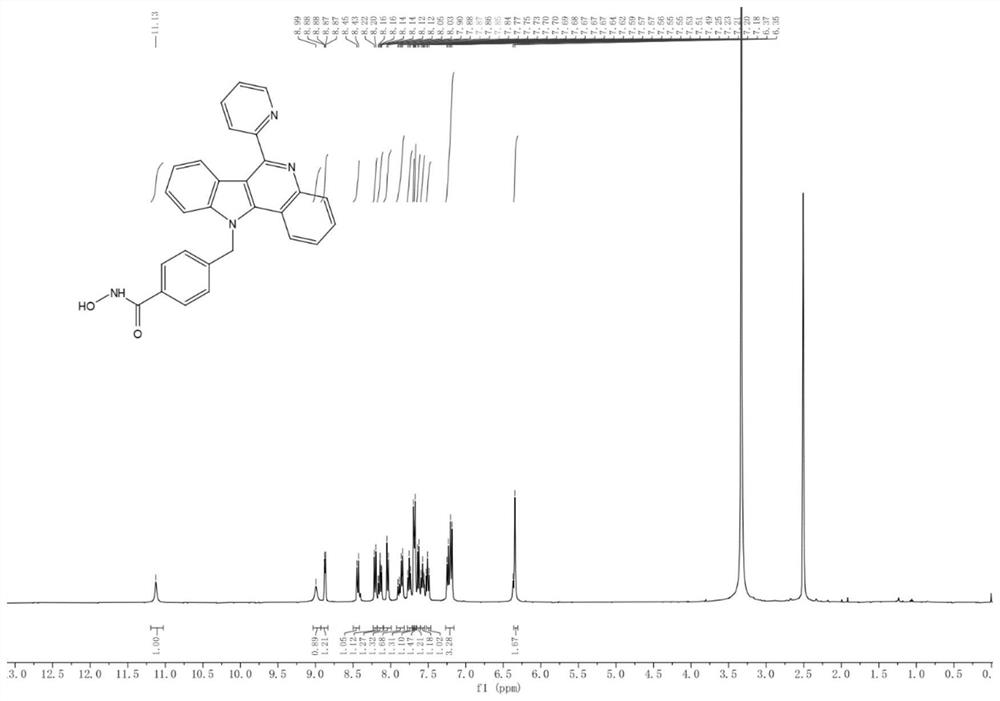
[0073] 4-((6-(4-Fluorophenyl)-11H indole[3,2-c]quinolin-11-yl)methyl)-N-hydroxybenzyl prepared in Example 1 was detected by MTT method In vitro antitumor activity of amides and N-hydroxy-4-((6-(pyridin-2-yl)-11H-indol[3,2-c]quinolin-11-yl)methyl)benzamide. The tumor cell lines were purchased from ATCC in the United States, and the anti-proliferative activity of the test compounds against human colorectal cancer cell line HCT-116, human breast cancer cell MCF-7, and melanoma cell B16-F10 was determined by standard MTT assay. -8 method to evaluate the cytotoxicity of human lymphoma Jurkat-T cell line. All cancer cell lines were cultured in RPMI-1640 medium s...
Embodiment 3
[0079] Example 3 In vitro study of HDAC inhibition
[0080] The in vitro anti-tumor activities of the compounds G3 and G12 of the present invention were tested by the following methods. These effects indicate that the compounds G3 and G12 of the present invention can be used to effectively inhibit HDAC6.
[0081] The specific test method is as follows:
[0082] The enzyme inhibitory activities of compounds G3 and G12 were determined by fluorescence analysis, wherein HDAC1 (#ab101661) and HDAC6 (#ab42632) enzymes were purchased from Abcam, HDAC3 (#BML-SE515-0050) was purchased from Enzo, HDAC8 (# H90-30H-05) was purchased from SignalChem. The buffer contains 25mmol / L Tris (pH 8.0), 1mmol / LMgCl 2 , 0.1mg / mL BSA, 137mmol / L NaCl, 2.7mmol / L KCl, of which HDAC (HDAC1, 7.2ng / well; HDAC3, 3.4ng / well; HDAC6, 15ng / well; HDAC8, 22ng / well), total volume to 40 μL. Compounds to be tested (10 dilution concentrations, followed by 500 μM, 125 μM, 31.25 μM, 7.81 μM, 1.95 μM, 0.49 μM, 0.12 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com