Production method of diaryl alkyne compound
A diaryl acetylene and diaryl acetylene technology, which is applied in the field of preparation of diaryl acetylene compounds, can solve problems such as difficulty in controlling the release rate of acetylene, difficulty in achieving large-scale application, difficulty in product separation, etc., and achieve the goal of overcoming the difficulty of reaction The effects of control, good scalability, and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
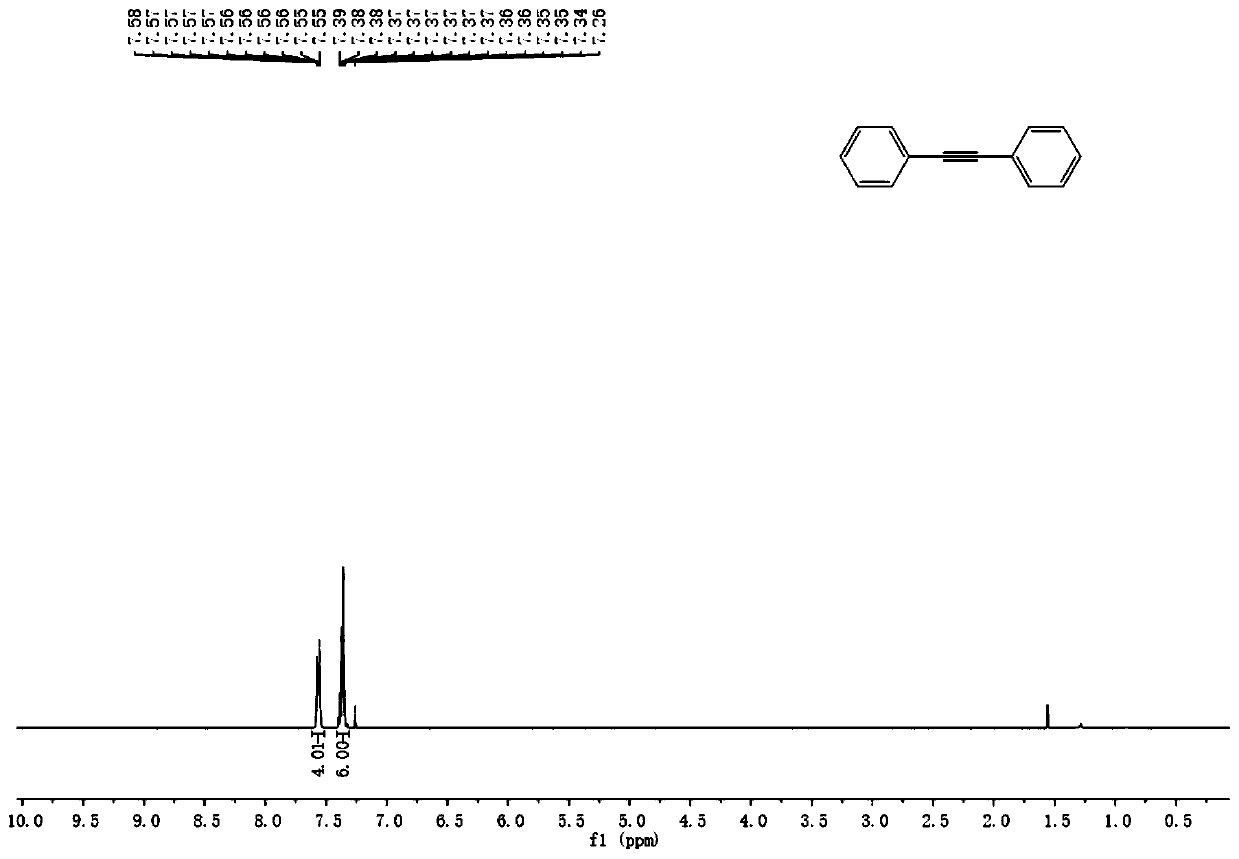
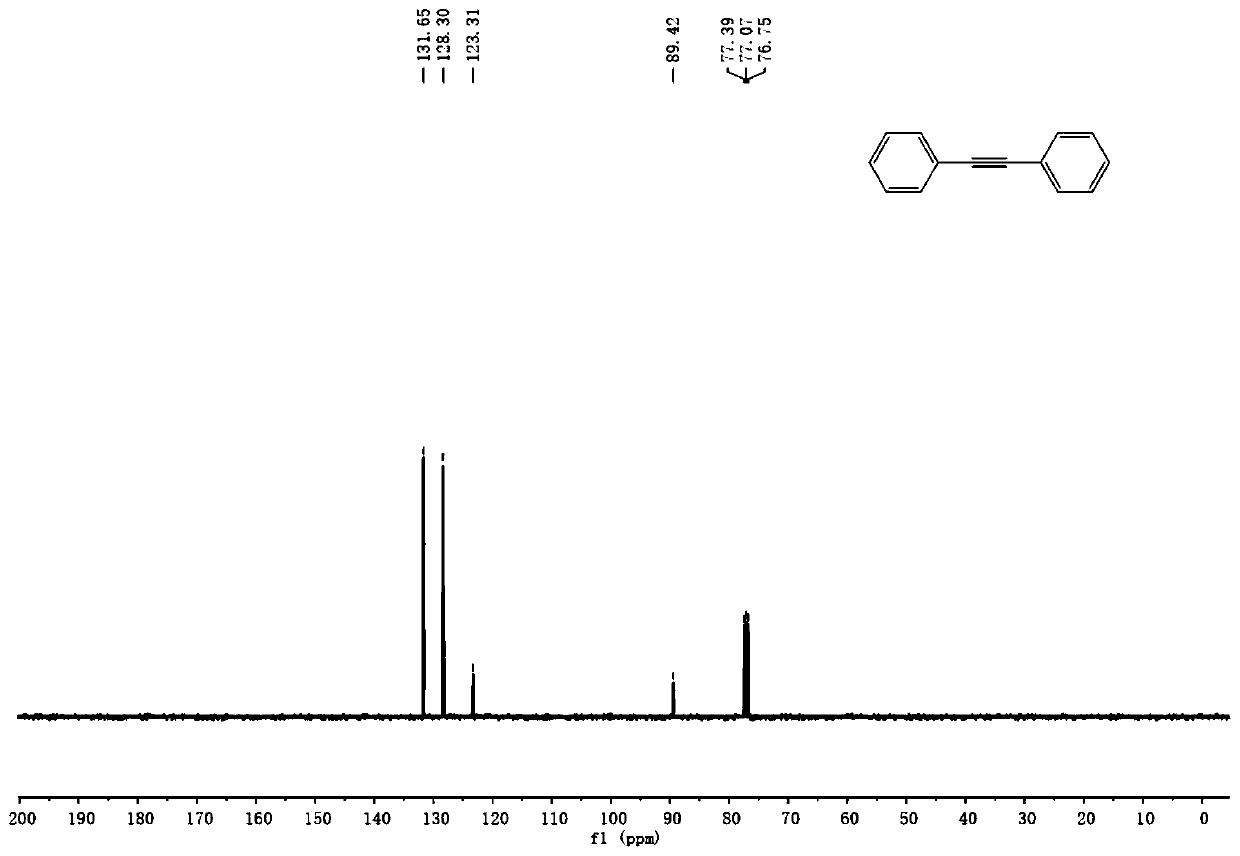
[0065] A kind of preparation method of diaryl alkyne compound of the present invention, its step is: under nitrogen protection, bromobenzene substrate, acetylenic alcohol, palladium catalyst, organic phosphine ligand and inorganic base are put into dry Schlenk tube Then add the solvent, heat the Schlenk tube to 100°C and stir the reaction for 12 hours, cool to room temperature and add saturated ammonium chloride solution to quench, then extract, separate and purify by column chromatography to obtain diarylethyne compounds. Wherein, the palladium catalyst is palladium acetate, the organic phosphine ligand is tert-butyldiphenylphosphine, the inorganic base is sodium tert-butoxide, and the solvent is tetrahydrofuran.
[0066] Wherein, the obtained product is a symmetrical diarylethyne compound or an unsymmetrical diarylethyne compound.
[0067] When the product is a symmetrical diaryl acetylene compound, its reaction formula is:
[0068]
[0069] When the product is a symmetr...
Embodiment 1
[0075] Under nitrogen atmosphere, 0.015mmol of palladium acetate, 0.03mmol of tert-butyldiphenylphosphine, 0.9mmol of sodium tert-butoxide and 0.165mmol of 2,5-dimethyl-3-hexyne-2,5-diol together Add it into a dry Schlenk tube, then add 0.3mmol bromobenzene and 2mL tetrahydrofuran, heat the reaction system to 100°C, stir for 12h and cool to room temperature; add 4mL saturated ammonium chloride solution to quench the reaction, add 8mL water and use Ethyl acetate was used for extraction, column chromatography separation, and purification to obtain diaryl alkyne compounds with a yield of 93%.
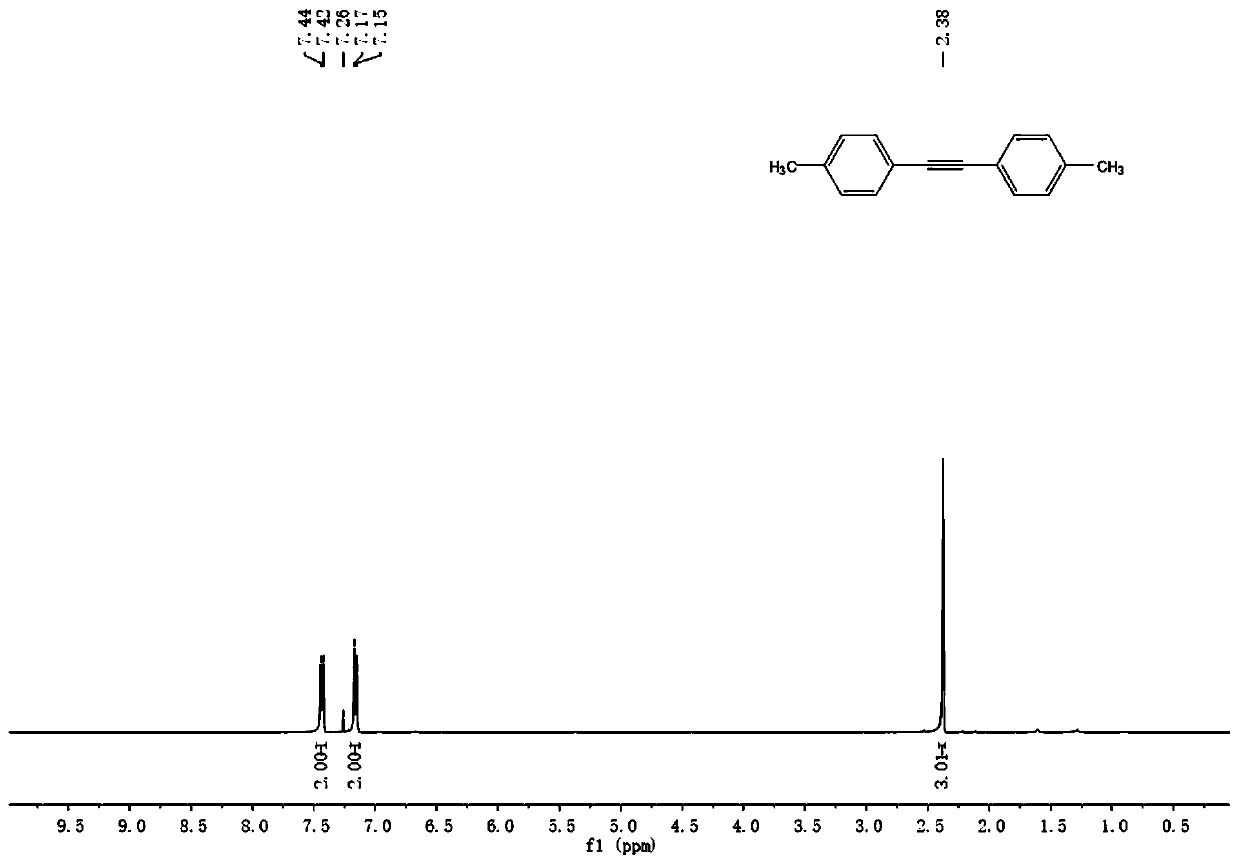
Embodiment 2-12
[0077] The difference from Example 1 is that different aryl bromide derivatives are used, other reaction conditions are the same as in Example 1, and the specific reactants and yields are shown in Table 1.
[0078] The general reaction formula of embodiment 1-12 is:
[0079]
[0080] Table 1 Synthesis of symmetrical diarylethynes by reaction of aryl bromide derivatives with 2,5-dimethyl-3-hexyne-2,5-diol
[0081]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com