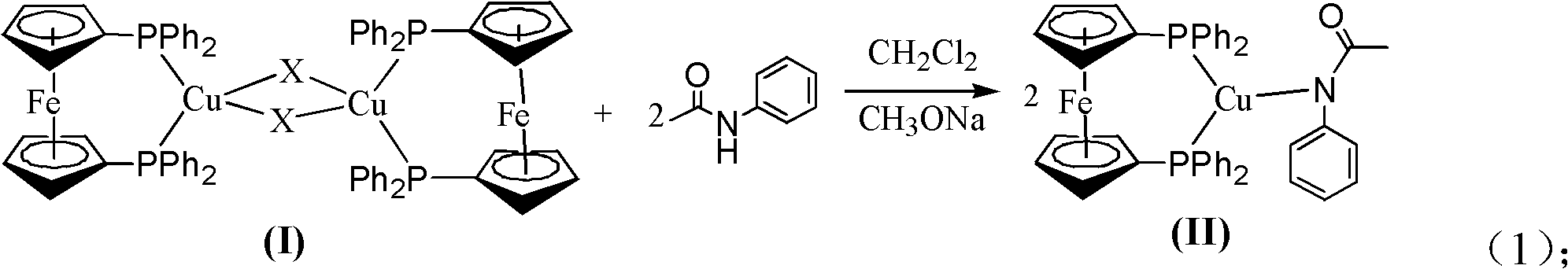
Method for preparing phenylamide compound
A technology for phenylamides and compounds is applied in the field of preparing phenylamides, and can solve the problems of polluted environment, high reaction temperature, long reaction time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment one: a kind of preparation has N, the method for N-diphenylacetamide comprises the following process steps:
[0027] Add iodobenzene (201mg, 1.00mmol), acetanilide (178mg, 1.21mmol), cuprous iodide (9.5mg, 0.050mmol, 5.0mol%), 1,1'-bis(diphenyl Phosphine) ferrocene (27.7mg, 0.050mmol, 5.0mol%), potassium phosphate (318mg, 1.50mmol), adding 5.0mL N, N-dimethylformamide; the reaction solution was reacted at 110°C for 12h, and the resulting The reddish-brown solution is filtered through a sand core funnel to remove solid impurities, and then separated through a 200-300 mesh silica gel column (the eluent is 1:5 ethyl acetate / petroleum ether), and after removing the solvent, N,N-diphenyl Acetamide, the structural formula is: The yield was 80%. 1H NMR (400MHz, CDCl3): 2.08 (s, 3H), 7.01-7.32 (m, 6H), 7.69 (d, J=7.2Hz, 4H). MS (m / z): 211 (M+).
Embodiment 2
[0028] Embodiment two: a kind of method for preparing N-phenyl-N-(4-methylphenyl) acetamide comprises the following process steps:
[0029] Add p-bromotoluene (171mg, 1.00mmol), acetanilide (270mg, 2mmol), cuprous bromide (14.3mg, 0.100mmol, 10.0mol%), 1,1'-bis(diphenyl Phosphine) ferrocene (110mg, 0.200mmol, 20.0mol%), potassium phosphate (318mg, 1.50mmol), add 5.0mL N,N-dimethylformamide, react the reaction solution at 80°C for 24h, the resulting reddish-brown The solution is filtered through a sand core funnel to remove solid impurities, and then separated by a 200-300 mesh silica gel column (eluent is 1:5 ethyl acetate / petroleum ether), and after removing the solvent, N-phenyl-N-(4 -Methylphenyl) acetamide, structural formula is:
[0030] Yield: 75%. 1H NMR (400 MHz, CDCl3): 2.08 (s, 3H), 2.32 (s, 3H), 7.12-7.40 (m, 9H). MS (m / z): 225 (M+).
Embodiment 3
[0031] Embodiment three: a kind of preparation N, the method for N-bis (4-methylphenyl) acetamide comprises the following processing steps:
[0032] Add p-bromotoluene (171mg, 1.00mmol), p-methylacetanilide (149mg, 1.00mmol), cuprous iodide (3.8mg, 0.020mmol, 2.0mol%), 1,1'-bis (Diphenylphosphine)ferrocene (11.1mg, 0.020mmol, 2.0mol%), potassium phosphate (318mg, 2.00mmol), add 5.0mL dimethyl sulfoxide, and react the reaction solution at 50°C for 24h, resulting in dark red The solution is filtered through a sand core funnel to remove solid impurities, and then separated by a 200-300 mesh silica gel column (eluent is 1:5 ethyl acetate / petroleum ether), and after removing the solvent, N, N-di(4- Methylphenyl) acetamide, structural formula is: The yield was 70%. 1H NMR (400 MHz, CDCl3): 7.48-7.02 (m, 8H), 2.33 (s, 6H), 2.08 (s, 3H). MS (m / z): 239 (M+).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com