Abiraterone acetate preparation method
A compound and a selected technology, applied in the preparation of carboxylate, the preparation of carboxylate, the preparation of organic compounds, etc., can solve the problems of not easy to clean, not suitable for large-scale production, and easy to attach activated carbon particles to the wall of the reactor.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
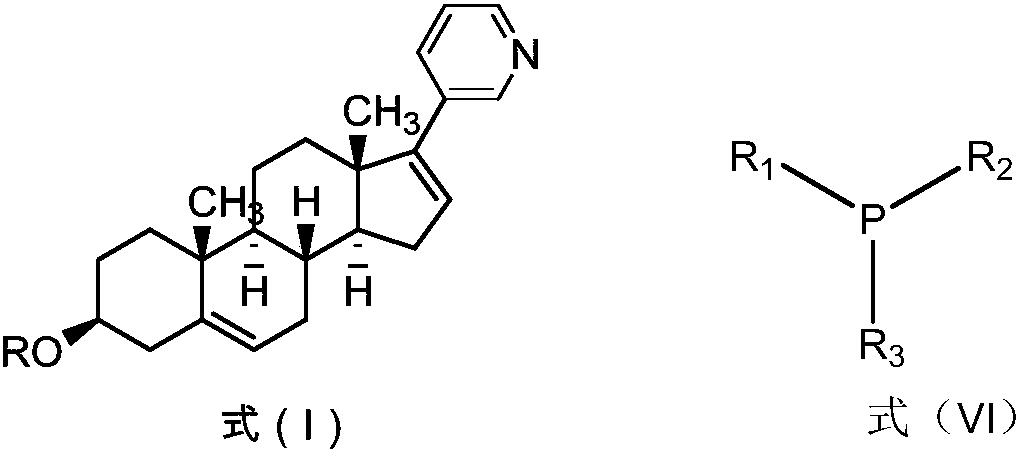
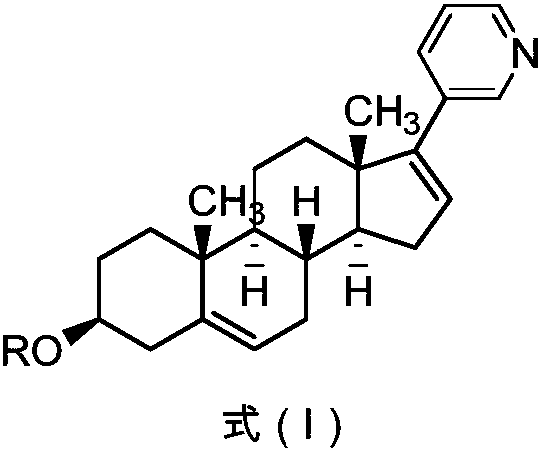
[0083] The preparation of embodiment 1 Abiraterone acetate
[0084] (1) Preparation of 3β-acetoxy-deoxyandrost-5-ene-17-hydrazone
[0085]
[0086] Add anhydrous ethanol (200kg), dehydroepiandrosterone acetate (60kg, 181.6mol), 80% hydrazine hydrate (15.0L, 209.2mol) and sulfuric acid (1.8L, 32.6mol) in sequence in a 1000L enamel reaction kettle. React until clarification, continue to react for 1h. Dichloromethane (300kg) and purified water (300kg) were added to wash and separate layers, the organic phase was washed with water, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated to dryness under reduced pressure, tetrahydrofuran (200kg) was added, stirred and dissolved to obtain a light yellow solution, That is, the tetrahydrofuran solution of 3β-acetoxy-deoxyandrost-5-ene-17-hydrazone was directly used in the next reaction.
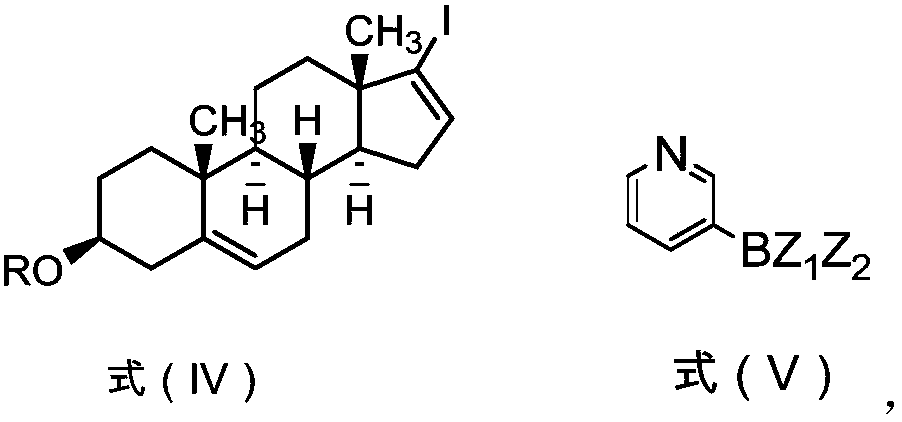
[0087] (2) Preparation of 17-iodoandrost-5,16-diene-3β-acetate
[0088]
[0089] Add tetrahydrofuran (300kg), iodine p...
Embodiment 2
[0099] The preparation of embodiment 2 Abiraterone acetate
[0100] (1) Preparation of 3β-acetoxy-deoxyandrost-5-ene-17-hydrazone
[0101]
[0102] Add absolute ethanol (10kg), dehydroepiandrosterone acetate (3kg, 9.09mol), 80% hydrazine hydrate (750ml, 10.5mol) and sulfuric acid (90ml, 1.63mol) successively in a 50L enamel reaction kettle, and react at room temperature to Clarify, continue to react for 1h. Dichloromethane (15kg) and purified water (15kg) were added to wash and separate the layers, the organic phase was washed with water, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated to dryness under reduced pressure, tetrahydrofuran (10kg) was added, stirred and dissolved to obtain a light yellow solution, That is, the tetrahydrofuran solution of 3β-acetoxy-deoxyandrost-5-ene-17-hydrazone was directly used in the next reaction.
[0103] (2) Preparation of 17-iodoandrost-5,16-diene-3β-acetate (formula IV-1)
[0104]
[0105] Add tetrahyd...
Embodiment 3
[0113] Example 3 0.22 μm membrane filtration to remove palladium
[0114] Add the crude abiraterone acetate (125g, palladium content: 53ppm), isopropanol (500ml) and activated carbon (3.0g) prepared in Example 2 to the 1L reaction bottle, and after refluxing to dissolve, pass through a 0.22 μm organic filter membrane Filter, replace the filtrate with a new filter membrane and filter twice, stir and crystallize at room temperature for 15 hours, filter, and blow dry at 80°C to obtain 95.0 g of abiraterone acetate as a white solid. HPLC purity: 99.8%, palladium content: 43ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com