Organic electroluminescence ethereal blue optical material, preparation method and application thereof
A coupling reaction and reaction process technology, applied in the field of organic electroluminescence materials, can solve the problems of limited electroluminescence applications and difficult regulation, and achieve high electric field and thermal stability, good amorphousness, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
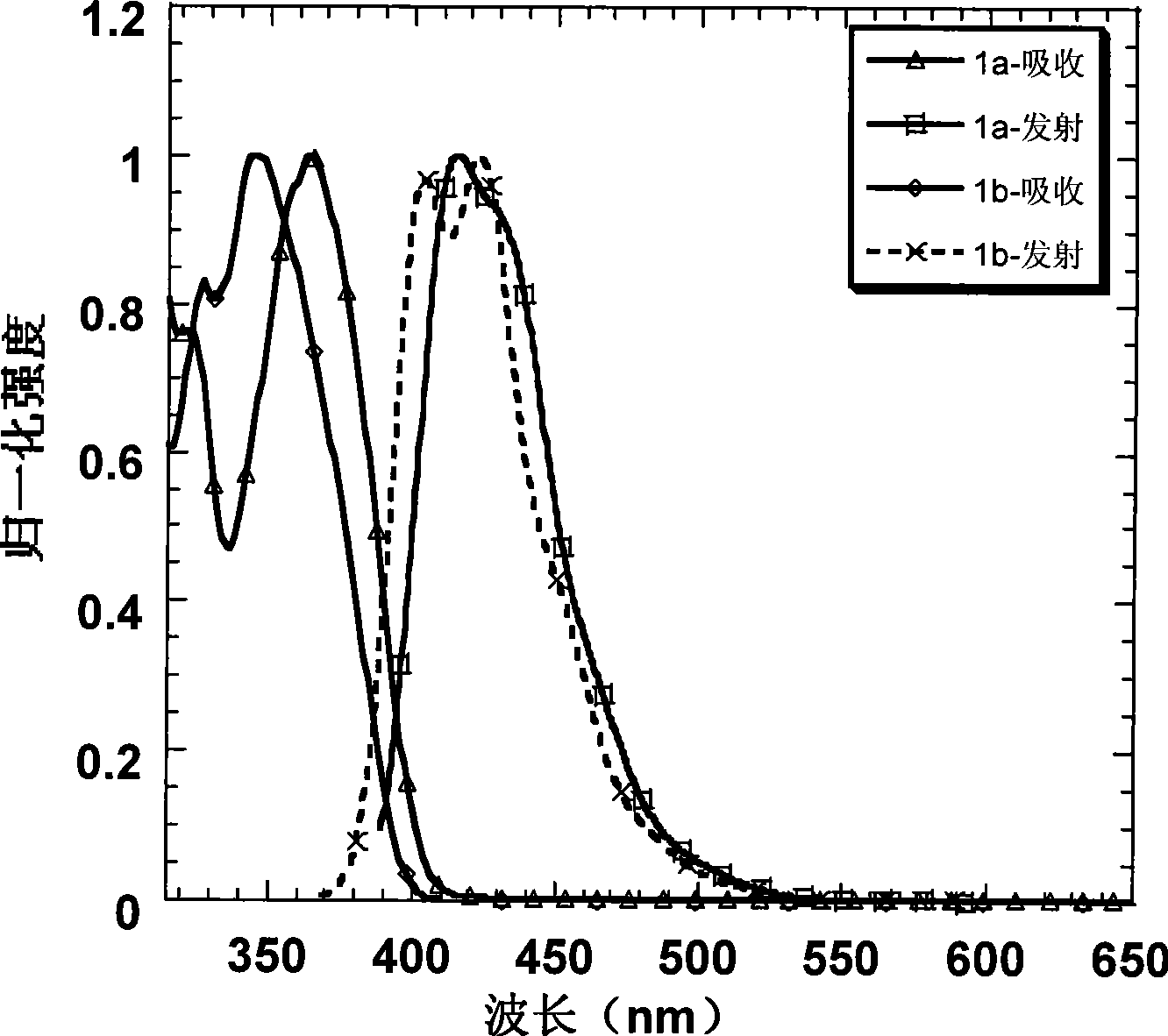
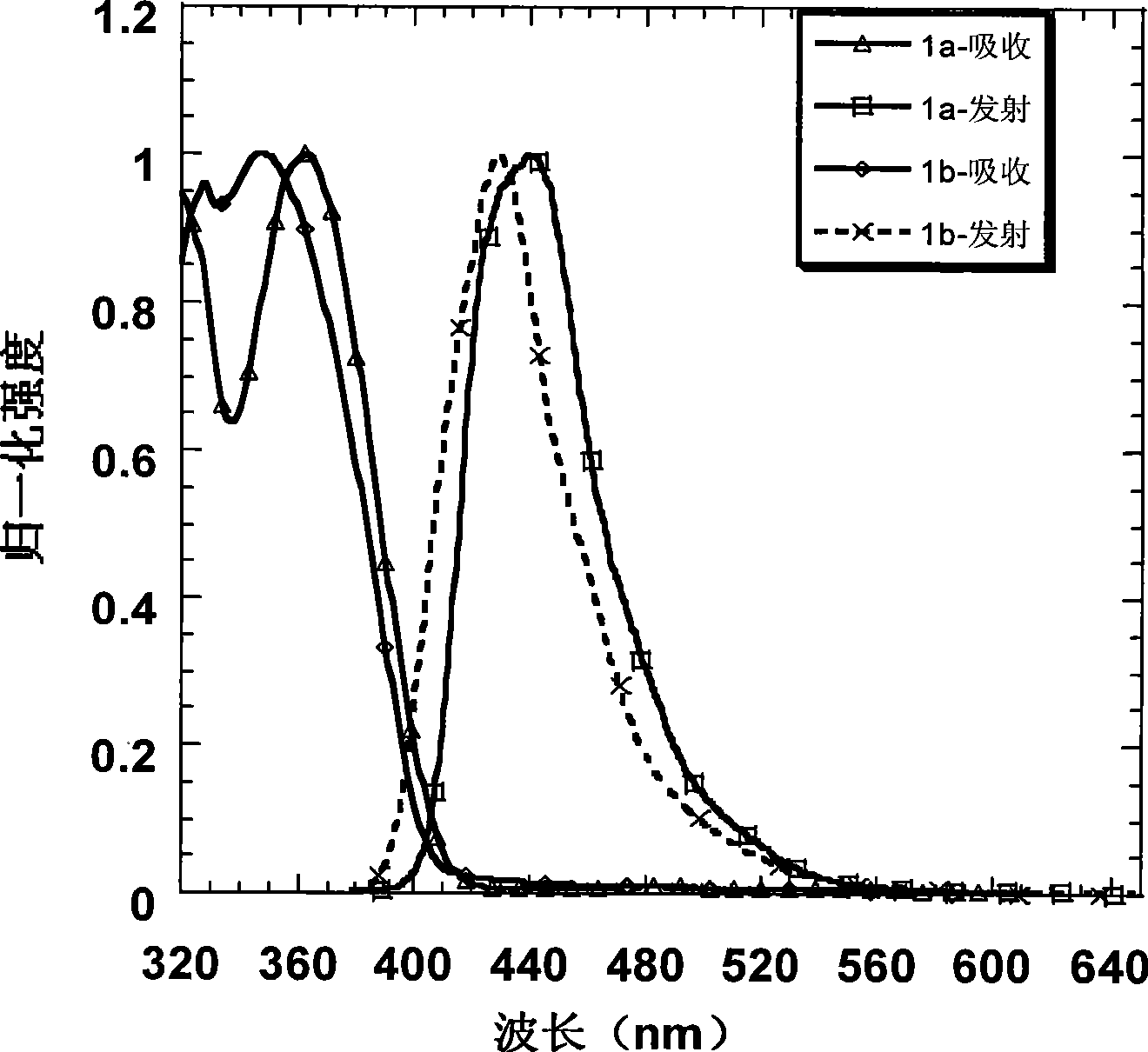
[0034] Embodiment 1: Preparation of 1a and its property determination
[0035] Use 2-bromo-3', 5-dimethoxybiphenyl to form a lithiated reagent under the action of tert-butyllithium at -78°C and attack the triketone compound. After acidification, a tertiary alcohol is obtained, and then in acetic acid and a catalytic amount Under the condition of methanesulfonic acid, a pale yellow solid was precipitated by ring closure. Suction filter the solid, wash with ethanol, and dry to obtain compound A, and then obtain intermediate 2a by bromination, and carry out Suzuki coupling reaction with spirobifluorene boronate under the catalysis of palladium catalyst to obtain the blue light material of the present invention 1a. The specific reaction process of preparing intermediate 2a is shown in the following formula:
[0036]
[0037] (1) Preparation of compound A:
[0038] Dissolve 2.93 g (10 mmol) of 2-bromo-3', 5-dimethoxybiphenyl in tetrahydrofuran (40 mL), and add a pentane solut...
Embodiment 2
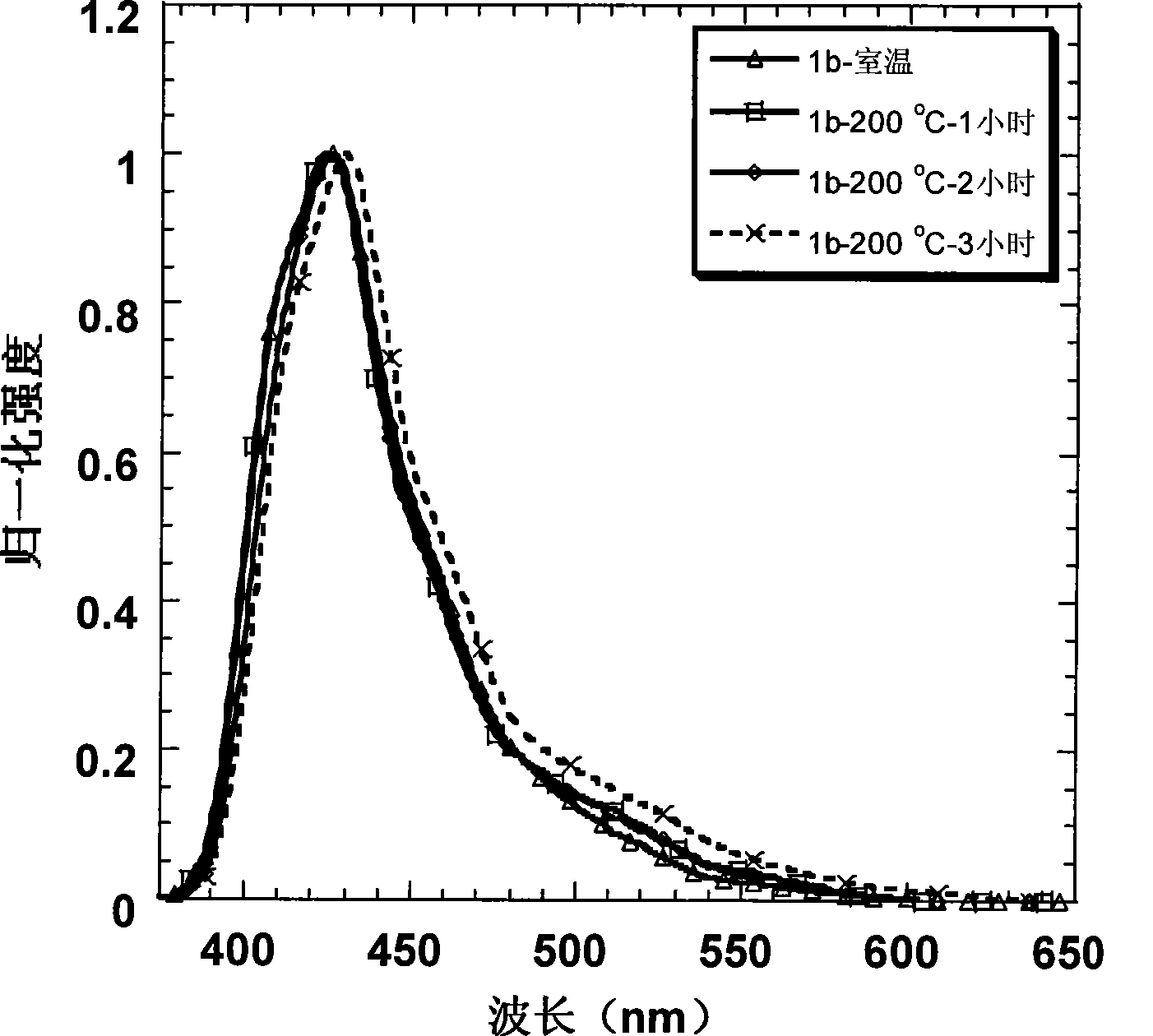
[0045] Embodiment 2: Preparation of 1b and its property determination
[0046] Starting from the industrial raw material m-bromoanisole, through the Friedel-Crafts acetylation of the para-position of the methoxy group, the two-step reaction of the acetyl trimerization promoted by silicon tetrachloride can obtain the tribromo compound in a higher yield; and then in Under the action of -78°C and tert-butyl lithium, the tribromo compound undergoes lithium-halide exchange to obtain a lithiated reagent. At this time, 2,7-dibromofluorenone is added to quench the carbanion, and the tertiary alcohol is obtained after acidification ; Finally, this tertiary alcohol is dissolved in dry dichloromethane, and boron trifluoride ether is added as a Lewis acid in a catalytic amount, and a white solid is precipitated in the system, which is the intermediate product 2b. After adding ethanol to quench the system After boron trifluoride, the solid was suction filtered, washed with ethanol several ...
Embodiment 3
[0059] Embodiment 3: device making
[0060] The typical device manufacturing process of the present invention is as follows: ITO (indium tin oxide) glass is ultrasonicated for ten minutes with acetone, alkaline washing solution, pure water (twice), and isopropanol, and then treated with ozone plasmar for 5 minutes. The hole injection layer PEDOT was spin-coated into a film with a thickness of 60 nm on the treated substrate, and the vacuum oven was kept at 80° C. overnight. Then the hole transport layer PVK (thickness 40nm) and the light emitting layer (1a or 1b50mg / mL toluene solution, thickness 70nm) were sequentially processed by spin coating respectively. The hole blocking layer TPBI (thickness 40nm) is vacuum evaporated on it (1-2A / s, 5×10 -4 pa), and finally Ba / Al (with a thickness of 4nm / 200nm) is vacuum-evaporated on it to form a complete device. The device structure is ITO / PEDOT(60nm) / PVK(40nm) / 1a or1b / TPBI(40nm) / Ba(4nm) / Al(200nm). Among them, the light-emitting lay...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com