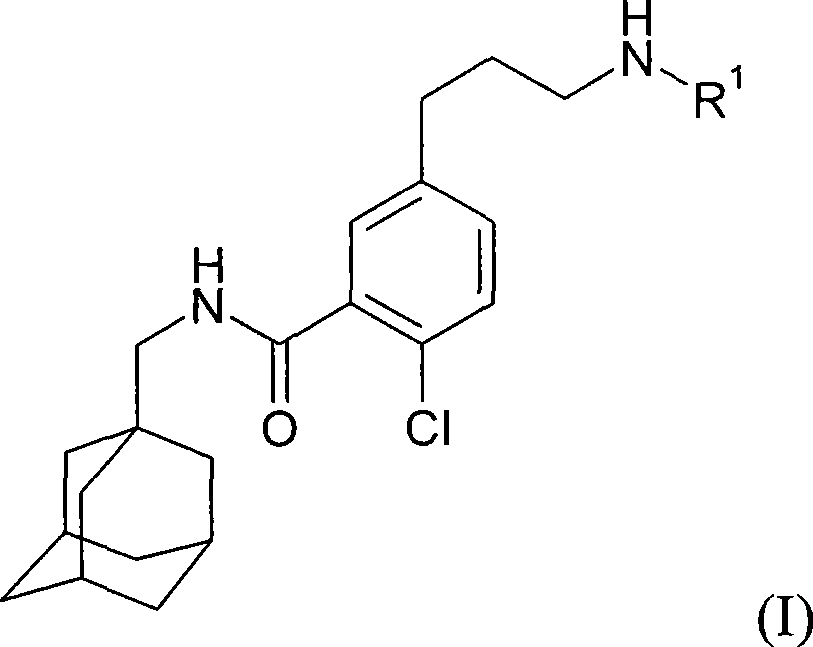
Process for preparing N-methyladamantyl derivatives by a palladium catalysed coupling reaction followed by reductive amination
An alkyl-reaction technology, applied in the field of preparing N-methyladamantyl derivatives by palladium-catalyzed coupling reaction followed by reductive amination, can solve expensive, unfavorable large-scale commercial synthesis, air sensitivity, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] The present invention will now be further explained by referring to the following exemplary embodiments.
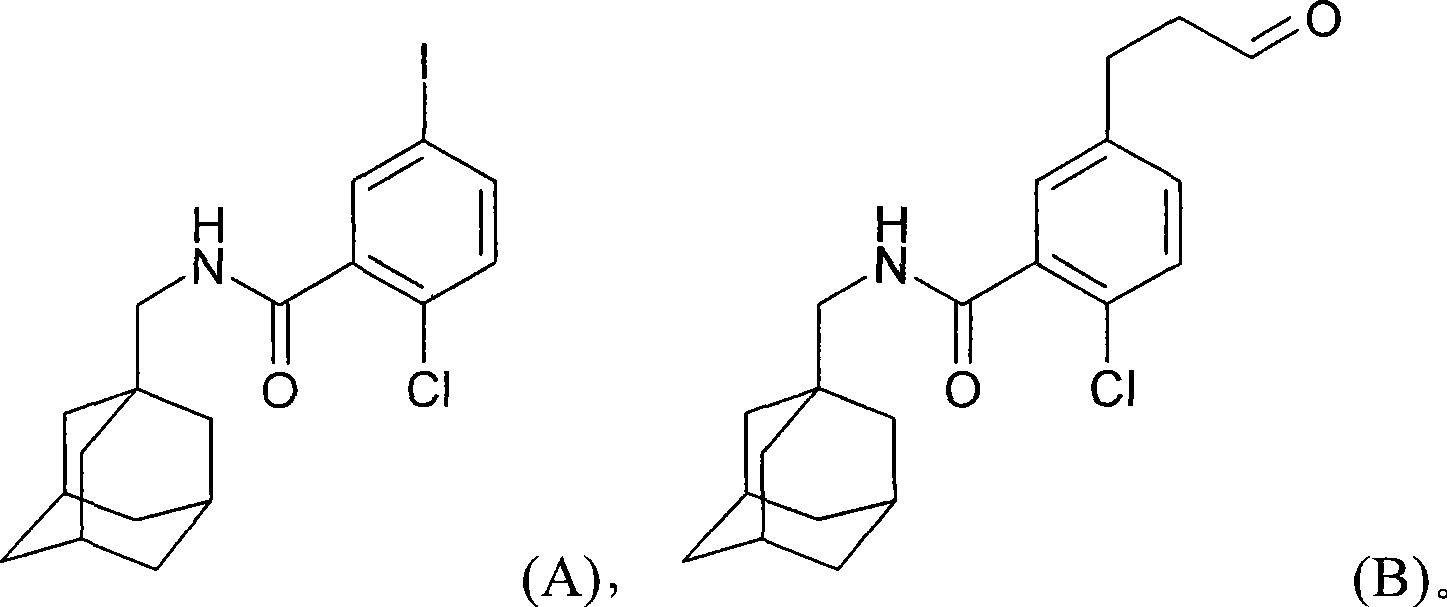
[0036] Preparation of 2-chloro-5-iodo-N-(tricyclic [3.3.1.1 3,7 ]Dec-1-ylmethyl)-benzamide (Compound A)
[0037] In an inert atmosphere (N 2 ) Add 5-iodo-2-chlorobenzoic acid (40.00g, 141.6mmol) to a 500ml reaction vessel, and then add Bu 4 NCl (0.40 g, 0.01 equivalent, 1.42 mmol) and toluene (80 ml, 2 volumes). The suspension was heated to 70-75°C, and then thionyl chloride (12.40 ml, 1.2 equivalents, 169.94 mmol) was added dropwise over 30-60 minutes. The resulting suspension was heated at 70-75°C for about 3 hours. The reaction was monitored by HPLC (the sample was quenched with MeOH). Once it was over, the reaction mixture, now a clear solution of 5-iodo-2-chlorobenzoyl chloride, was cooled to 20-25°C.
[0038] Add 1-adamantane methylamine.HCl (28.56g, 1.0 equivalent, 141.62mmol), toluene (40ml, 1.0 volume) and 5M NaOH aqueous solution (84.96ml, 3.0 equivalen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com