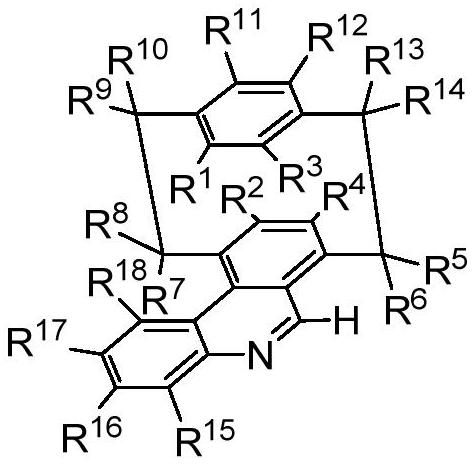
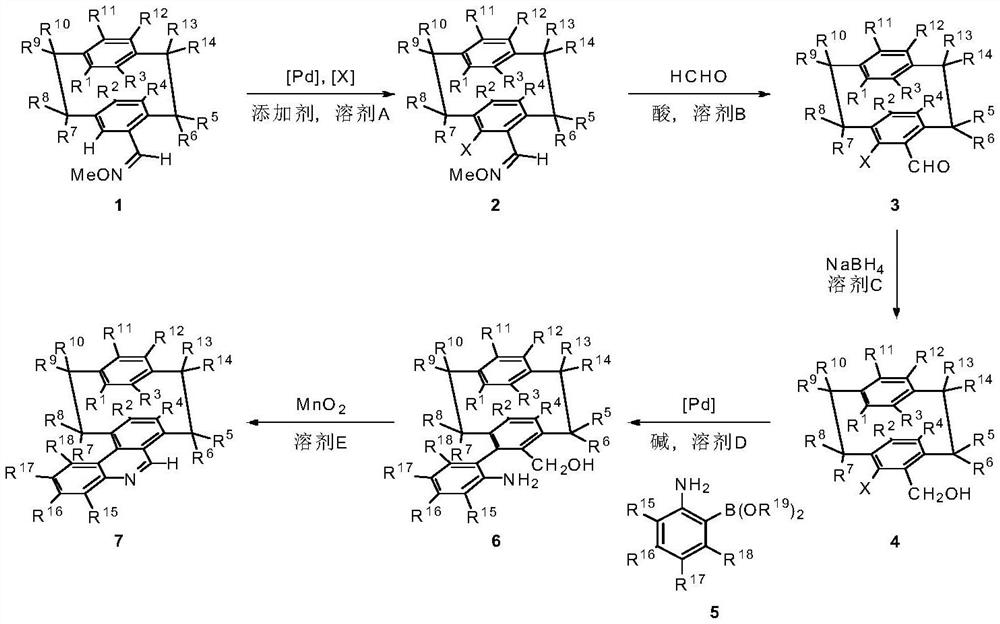
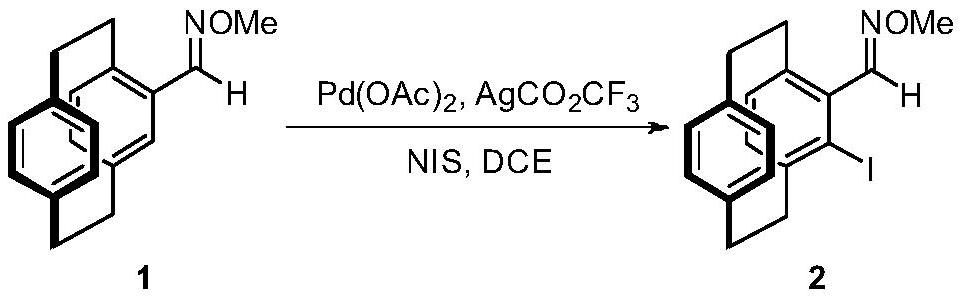A class of nad(p)h mimetics with a chiral cycloaryl alkanoline skeleton and its synthesis method and application
A synthetic method and simulant technology, applied in organic chemical methods, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve problems such as poor stability, difficulty, cumbersome synthesis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the synthesis of compound 2
[0047]
[0048] Under nitrogen protection, compound 1 (1.900g, 7.16mmol), NIS (1.933g, 8.59mmol), Pd(OAc) were added to a 250mL reaction flask 2 (0.322g, 1.43mmol), AgCO 2 CF 3 (0.316g, 1.43mmol), 120mL 1,2-dichloroethane. The temperature was raised to 110°C and the reaction was stirred. Diatomaceous earth assisted filtration, and column chromatography gave 2.269 g of a yellow solid, with a yield of 81%. 1 HNMR (400MHz, CDCl 3 )δ8.05(d,J=1.1Hz,1H),7.01(d,J=7.8Hz,1H),6.59(dd,J=9.1,4.5Hz,2H),6.52(dd,J=12.6,4.8 Hz,2H),6.46(d,J=7.7Hz,1H),4.03(d,J=0.8Hz,3H),3.84–3.73(m,1H),3.46(t,J=10.6Hz,1H), 3.21–2.94(m,5H),2.87(m,1H); 13 C NMR (100MHz, CDCl 3 )δ153.5,143.8,140.7,139.6,138.8,135.0,134.3,133.5,133.2,132.9,130.0,128.6,110.1,62.2,39.98,35.0,34.7,33.1. HRMS: Calculated for C 18 h 19 INO[M+H] + 392.0506,found 392.0507.
Embodiment 2
[0049] Embodiment 2: the synthesis of compound 2
[0050]
[0051] Under nitrogen protection, compound 1 (200mg, 0.76mmol), NBS (163mg, 0.91mmol), Pd(OAc) were added to the 50mL reaction flask 2 (34mg, 0.15mmol), AgCO 2 CF 3 (34mg, 0.15mmol), 15mL 1,2-dichloroethane. The temperature was raised to 110°C and the reaction was stirred. Diatomaceous earth assisted filtration, and column chromatography gave 145 mg of white solid, yield 55%.
Embodiment 3
[0052] Embodiment 3: the synthesis of compound 3
[0053]
[0054] Compound 2 (1.200 g, 3.07 mmol), p-benzenemethanesulfonic acid monohydrate (1.168 g, 6.14 mmol), aqueous formaldehyde (4.6 mL, 61.40 mmol), 10 mL tetrahydrofuran and 1 mL water were added to a sealed 25 mL tube. The temperature was raised to 130° C., and the reaction was carried out for 72 hours. Cool to room temperature, add 10mL H 2 O, dichloromethane extraction, anhydrous Na 2 SO 4 Drying and column chromatography gave 1.046 g of a yellow solid with a yield of 94%. 1 H NMR (400MHz, CDCl 3 )δ9.79(s,1H),6.98(dd,J=7.9,1.8Hz,1H),6.68–6.56(m,3H),6.49(dd,J=7.9,1.8Hz,1H),6.38(dd ,J=7.9,1.8Hz,1H),3.88(m,1H),3.63–3.49(m,1H),3.25–3.15(m,3H),3.09–2.96(m,2H),2.81(m,1H ). 13 C NMR (100MHz, CDCl 3 )δ197.8,144.6,144.2,139.8,138.8,137.0,135.4,135.3,133.2,132.8,131.1,128.8,111.1,39.2,35.0,34.3,33.1. HRMS: Calculated for C 17 h 16 IO[M+H] + 363.0240,found 363.0244.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com