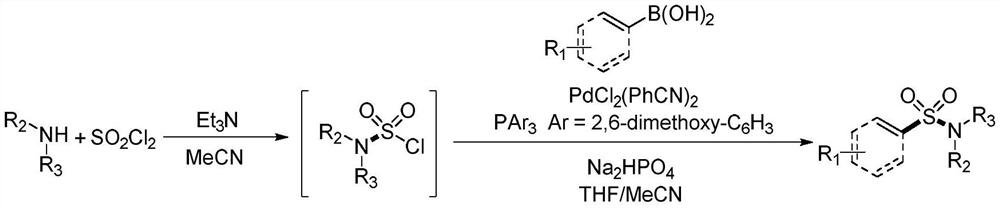
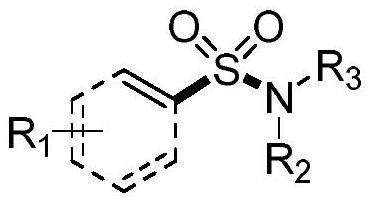
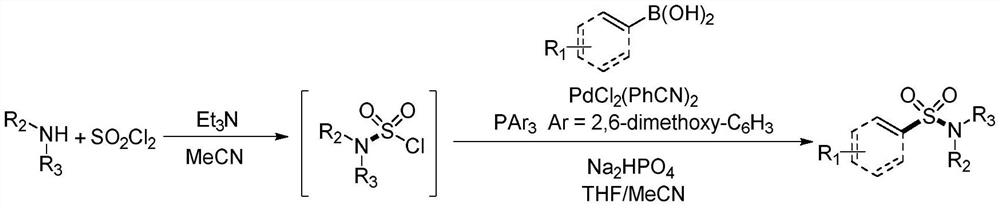
Synthesis method of sulfonyl secondary amine compound
A technology of secondary sulfonyl amine and synthesis method, which is applied in the field of synthesis of secondary sulfonyl amine compounds, can solve the problems of relying on self-synthesis, limited sulfonyl chloride reagents and the like, and achieves the effect of good guiding significance and application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] At room temperature, place the dry test tube reaction tube in high-purity nitrogen or argon to replace the gas, so that the system is in anhydrous and oxygen-free conditions, then add 1.0mL of acetonitrile and 0.5mmol of sulfuryl chloride and cool to 0°C, and then dropwise add 0.2 mmol of morpholine and 0.53 mmol of triethylamine were placed in a heating device at 50° C. and stirred for 0.5 hours.
[0035] Take another reaction tube, add 0.02mmol of bisbenzonitrile palladium dichloride, 0.04mmol of tris(2,6-dimethoxyphenyl)phosphine, 0.6mmol of disodium hydrogen phosphate and 0.4mmol of 2 -Naphthylboronic acid, place the reaction tube in high-purity nitrogen or argon to replace the gas, so that the system is in anhydrous and oxygen-free conditions, add 1.0mL tetrahydrofuran and 0.5mL acetonitrile, and then add the mixed solution obtained in the previous step with a syringe, and the obtained The mixture was stirred in a heating apparatus at 70°C for 16 hours....
Embodiment 2
[0038]
[0039] At room temperature, place the dry test tube reaction tube in high-purity nitrogen or argon to replace the gas, so that the system is in anhydrous and oxygen-free conditions, add 1.5mL of acetonitrile and 0.5mmol of sulfuryl chloride and cool to 0 ° C, and then dropwise add 0.2 mmol of morpholine and 0.5 mmol of triethylamine were placed in a heating device at 30° C. and stirred for 0.5 hours.
[0040] Another reaction tube was taken, and 0.01 mmol of bisbenzonitrile palladium dichloride, 0.02 mmol of tris(2,6-dimethoxyphenyl) phosphine, 0.6 mmol of disodium hydrogen phosphate and 0.4 mmol of 4 -Bromophenylboronic acid, place the reaction tube in high-purity nitrogen or argon to replace the gas, so that the system is in anhydrous and oxygen-free conditions, then add 1.0mL tetrahydrofuran and 0.5mL acetonitrile, then add the mixed solution obtained in the previous step with a syringe, and The resulting mixture was stirred in a 70°C heating apparatus for 16 ho...
Embodiment 3
[0043]
[0044] At room temperature, place the dry test tube reaction tube in high-purity nitrogen or argon to replace the gas, so that the system is in anhydrous and oxygen-free conditions, then add 3.0mL of acetonitrile and 0.9mmol of sulfuryl chloride and cool to 0°C, then dropwise add 0.6 mmol of morpholine and 1.6 mmol of triethylamine were placed in a heating device at 50° C. and stirred for 1 hour.
[0045] Another reaction tube was taken, and 0.009 mmol of bisbenzonitrile palladium dichloride, 0.018 mmol of tris(2,6-dimethoxyphenyl) phosphine, 1.5 mmol of disodium hydrogen phosphate and 1.0 mmol of 4 -Vinylphenylboronic acid, place the reaction tube in high-purity nitrogen or argon to replace the gas, so that the system is in anhydrous and oxygen-free conditions, add 3.0mL tetrahydrofuran and 1.5mL acetonitrile, and then add the mixed solution obtained in the previous step with a syringe, The resulting mixture was stirred in a heating apparatus at 50°C for 24 hours....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com