Preparation method of diallyl-terminated polyether and diallyl-terminated polyether
A bisallyl-capped and polyether technology is applied in the field of preparation of bisallyl-terminated polyether and bisallyl-terminated polyether, which can solve the problem of low molecular weight, unfavorable sealant application and widening of molecular weight distribution. and other problems to achieve the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
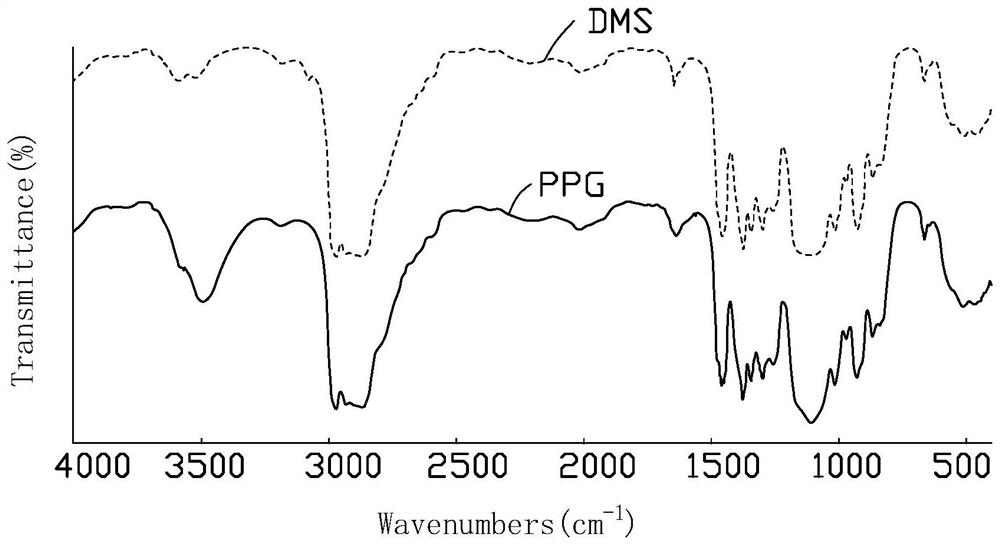
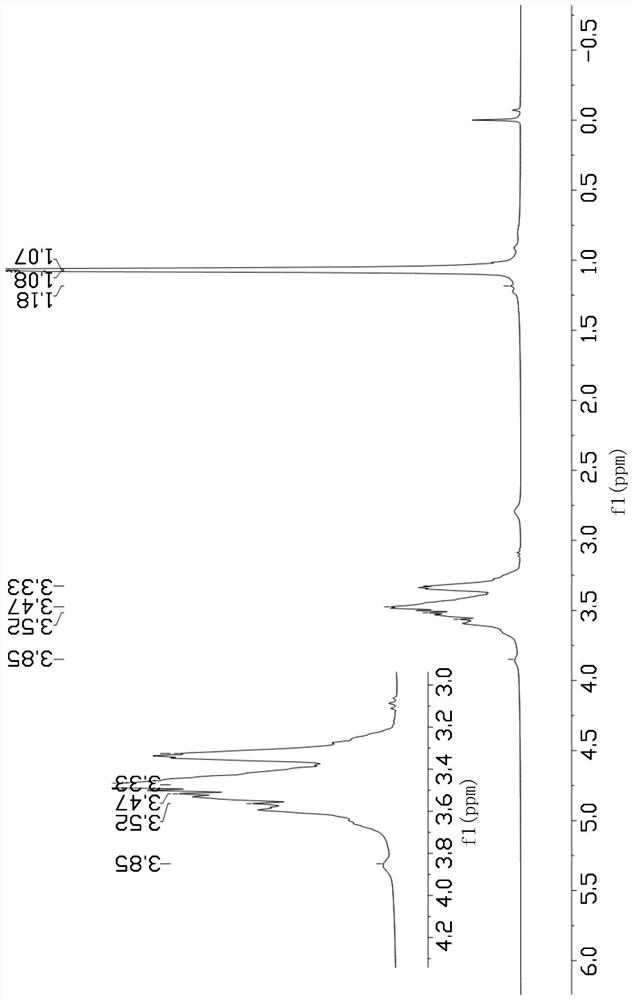
Image
Examples
preparation example Construction
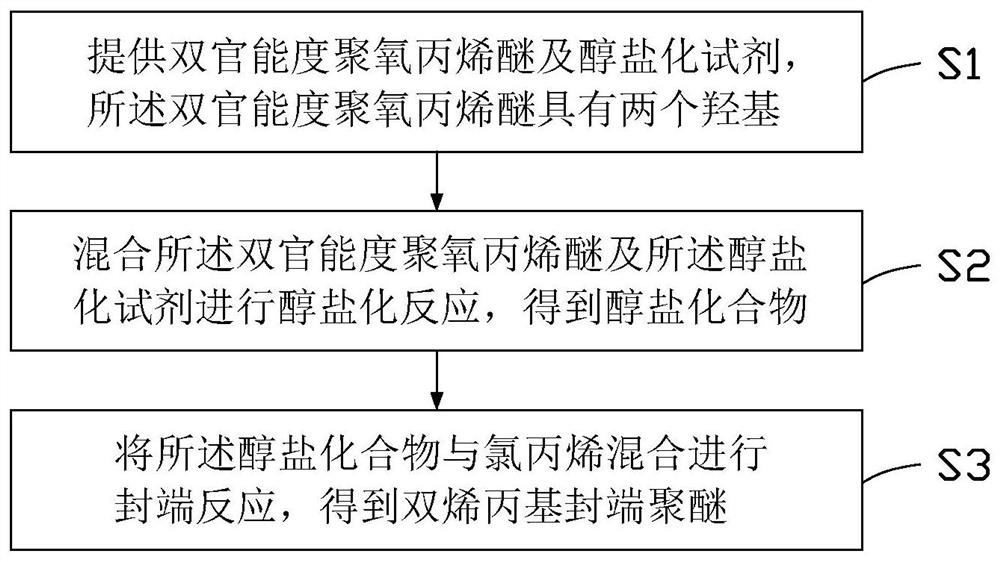
[0034] see figure 1 , a method for preparing a high molecular weight diallyl-terminated polyether provided in an embodiment of the present invention, comprising the following steps:
[0035] Step S1: providing a difunctional polyoxypropylene ether and an alkoxide reagent, the difunctional polyoxypropylene ether having two hydroxyl groups.
[0036] The molecular formula of the difunctional polyoxypropylene ether is:
[0037]
[0038] Wherein, n is a natural number, and the molecular weight of the difunctional polyoxypropylene ether is 4000-20000.
[0039] The difunctional polyoxypropylene ether is a polyoxypropylene ether having two functional groups capable of participating in the subsequent alkoxide reaction, that is, the two hydroxyl functional groups in the above molecular formula. That is, the difunctional polyoxypropylene ether is polyether diol.
[0040] The alkoxide reagent includes at least one of potassium tert-butoxide, sodium hydride, potassium methylate and p...
Embodiment 1
[0066] Add 1000 g of difunctional polyoxypropylene ether with a molecular weight of 4000 into a reaction kettle with a volume of 2 L, and add 22 g of sodium hydride with a mass fraction of 60% as an alkoxide reagent.
[0067] After sealing the reactor and replacing it with nitrogen for 3 times, pre-react the polyether diol and sodium hydride at room temperature for 30 minutes; then slowly raise the temperature to 130°C, stir together during the heating process, and maintain the reaction pressure at 0.01MPa -0.1MPa, in order to remove the gas generated during the reaction, the reaction time of alkoxide is 6h.
[0068] Lower the temperature of the polyether polyol salt formed after the alkoxide reaction to 30°C-40°C, add 42.1g of propylene chloride, increase the pressure in the reactor to 300kPa, and raise the temperature in the reactor to 60°C- After reacting for 6 hours at 70°C, a turbid light yellow reaction intermediate was obtained.
[0069] Add 22g of phosphoric acid with...
Embodiment 2
[0071] The difference from Example 1 is: the molecular weight of polyether polyol is 8000, the mass of sodium hydride is 11g; the mass of allyl chloride is 21g; the mass of phosphoric acid is 11g.
[0072] Other steps are the same as those in Embodiment 1, and will not be repeated here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com