Method for constructing zebra fish asap1b gene-knockout mutant by using CRISPR/Cas9 technology
A gene knockout and zebrafish technology, which can be applied to other methods of inserting foreign genetic materials, genetic engineering, recombinant DNA technology, etc., can solve problems such as the absence of zebrafish asap1b gene mutants and difficulty in transformation, and achieve a knockout process Simple and fast, high medical research value effect
Inactive Publication Date: 2019-12-06
SHANXI UNIV +1
View PDF1 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
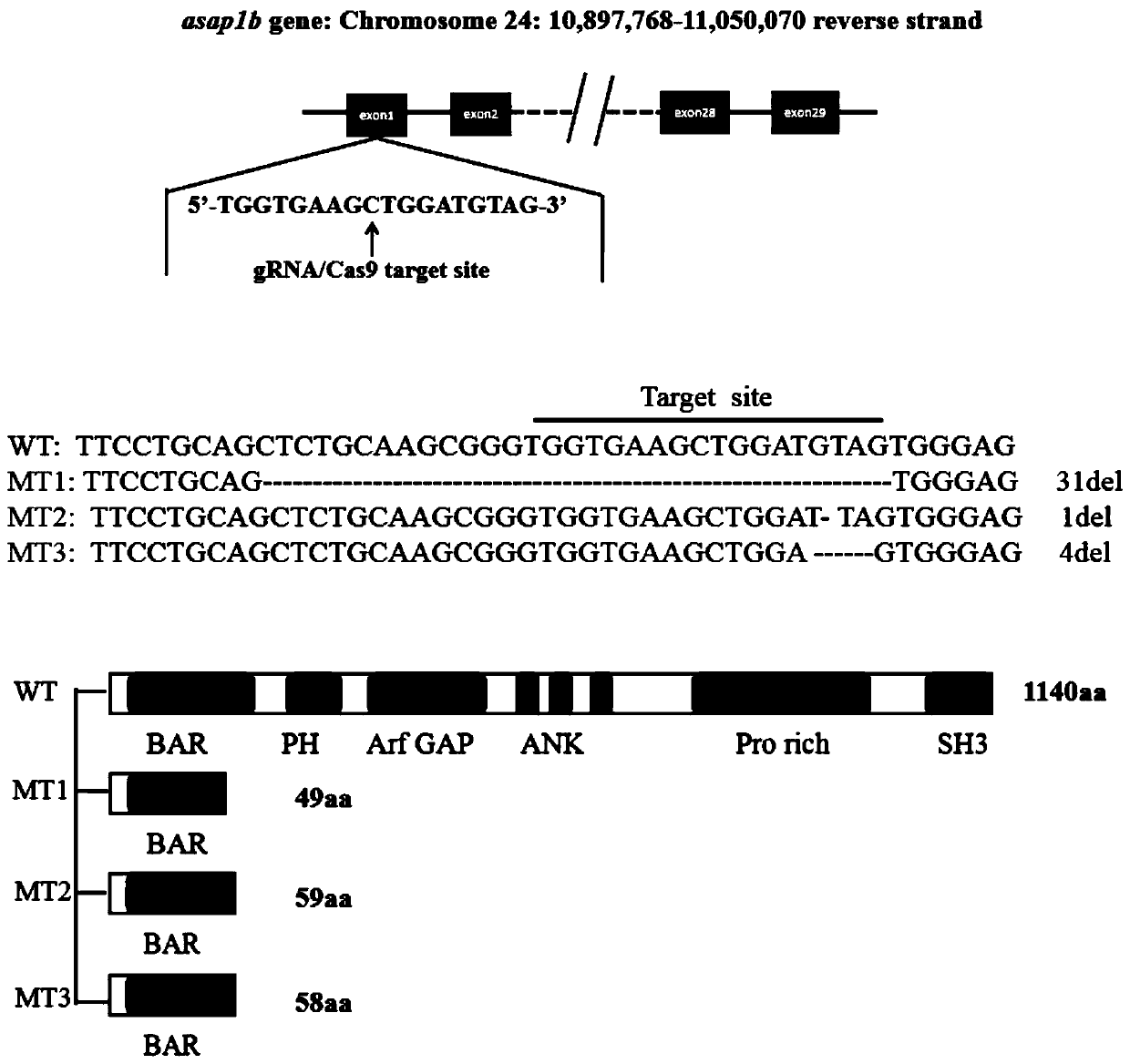
However, there are few reports on transforming tuberculosis susceptibility genes in zebrafish models to study the pathophysiology of tuberculosis, and there is no report on the use of gene editing technology to generate zebrafish asap1b gene mutants. In addition, the number of asap1b transcripts is as high as 6. CRISPR / Cas9 technology makes modification of asap1b more difficult
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
[0037] Materials involved in the present invention, equipment are specifically as follows:
[0038] 1 Materials and Equipment
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
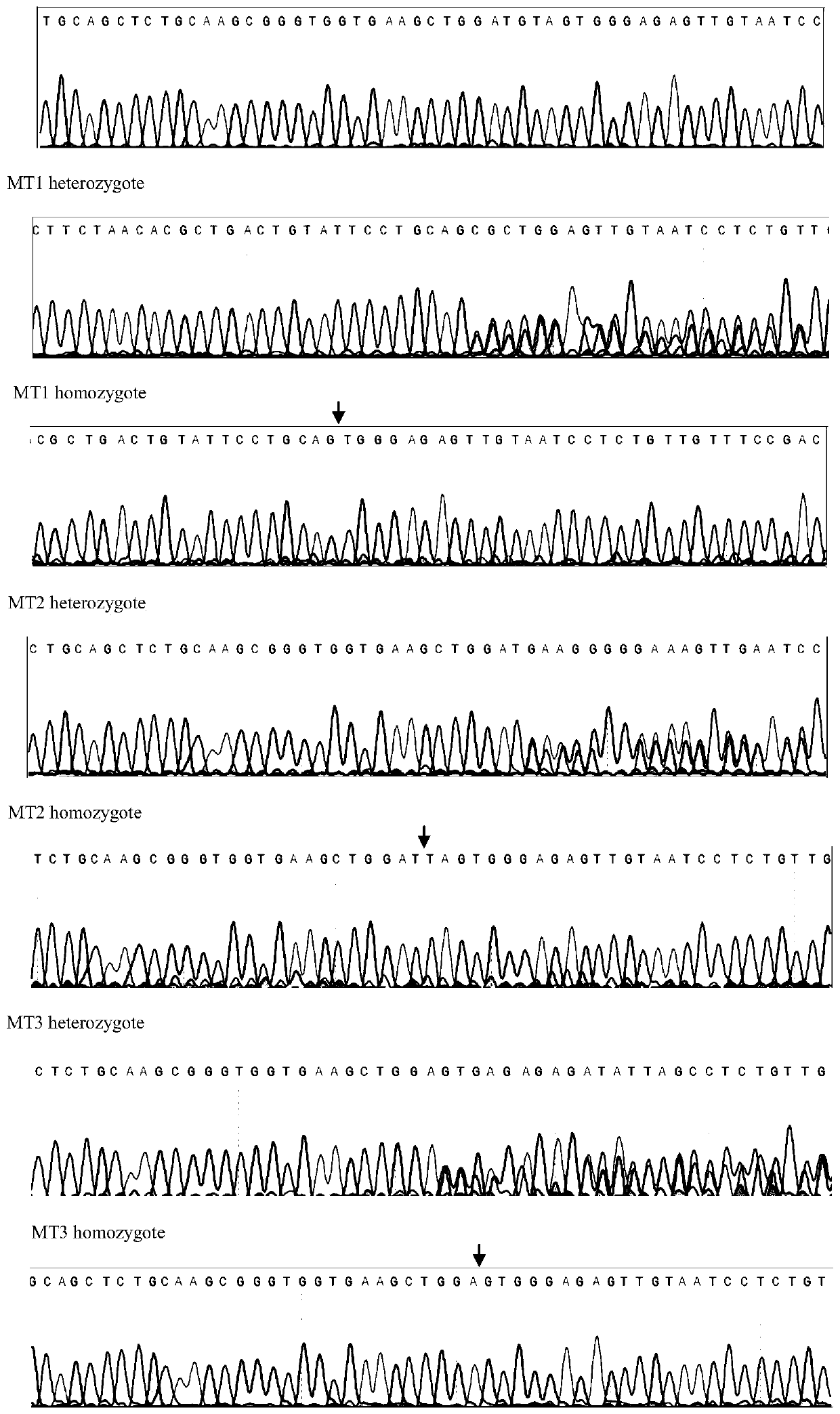
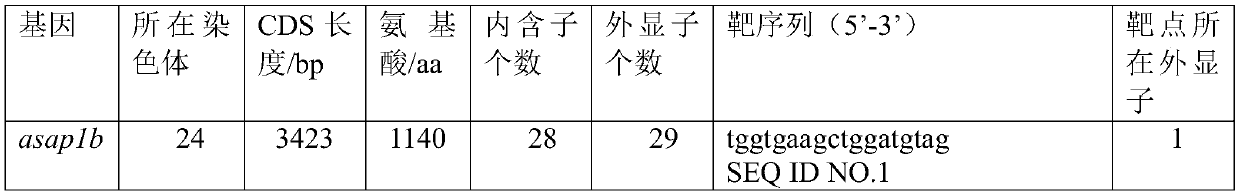
The invention discloses a method for constructing a zebra fish asap1b gene-knockout mutant by using a CRISPR / Cas9 technology. The method specifically comprises the following steps: determining the position of an asap1b-gRNA-Cas9 target site where the asap1b gene is knocked out, carrying out in-vitro transcription to obtain asap1b-gRNA, introducing the asap1b-gRNA and Cas9mRNA into zebra fish fertilized eggs, and carrying out screening culture to obtain the stably-inherited asap1b gene-knockout mutant. According to the invention, according to an selected section of high-efficiency targeting sequence located at the first exon of an asap1b gene, the asap1b gene in the zebra fish is knocked out by using the CRISPR / Cas9 technology, and the asap1b gene-knockout zebra fish capable of being stablyinherited is generated. Three mutation types of asap1b gene-knockout zebra fish strains are obtained in total, Asap1b protein translation is terminated in advance in all the strains, and an animal model foundation is laid for researching functions of the asap1b gene.
Description
technical field [0001] The invention relates to the technical field of genetically engineered zebrafish mutants, in particular to a method for constructing zebrafish asap1b gene knockout mutants using CRISPR / Cas9 technology. Background technique [0002] The CRISPR / Cas system is an acquired immune defense system that exists in bacteria and archaea. When bacteria are infected by viruses, they will leave foreign gene fragments in their genomes as "memory antibodies" to identify re-invading viruses. . The CRISPR / Cas system consists of a CRISPR (Clustered regularly interspaced short palindromicrepeat) element and a gene encoding a series of Cas proteins, wherein the CRISPR element consists of multiple identical repeat sequences (repeats) and different spacers (spacers) alternately arranged. The working principle of the system is: bacteria rely on Cas protein to capture exogenous nucleic acid fragments and insert them into the spacer sequence of the genome CRISPR element, so tha...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C12N15/90C12N9/22A01K67/027
CPCA01K67/0275A01K2217/075A01K2227/40A01K2267/0337C12N9/22C12N15/902
Inventor 崔佳吴长新赵仲华温炟王玉环
Owner SHANXI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com