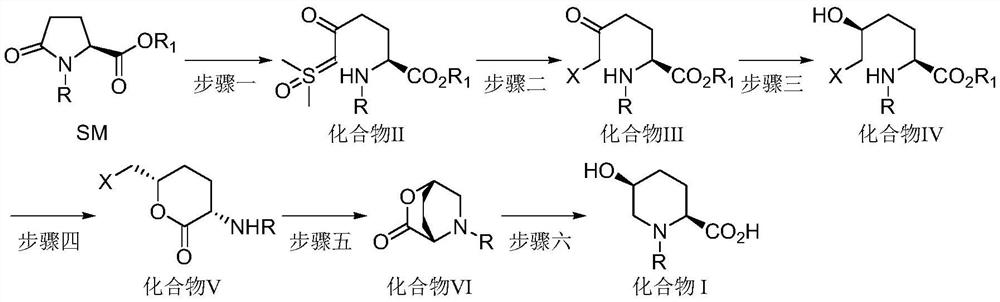
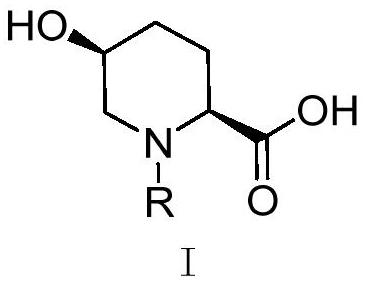
Synthetic method of avibactam intermediate (2s, 5s)-n-protecting group-5-hydroxyl-2-carboxylic acid piperidine
A technology of piperidine formate and a synthetic method, which is applied in the field of drug synthesis, can solve the problems of unsuitability for industrial production, insufficient total yield, high cost, etc., and achieve the effects of easy control of impurities, low price, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
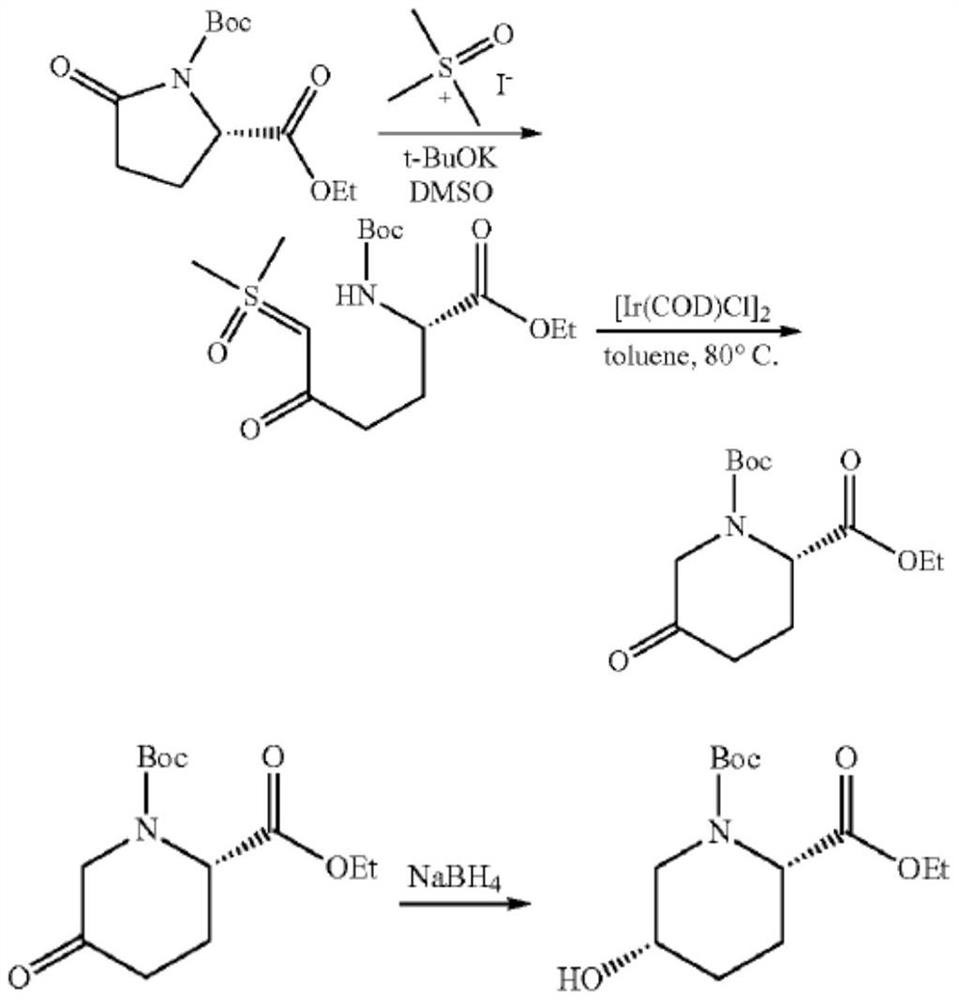
[0046] 1. Synthesis of (S)-ethyl-2-[(tert-butoxycarbonyl)amino]-6-(dimethylsulfinyl)-5-oxohexanoate (compound II)
[0047]
[0048] Add tetrahydrofuran (540mL) and dimethyl sulfoxide (650mL) to the reaction flask in turn, stir trimethylsulfoxide iodide (154g) to completely dissolve, and slowly add potassium tert-butoxide (78.6g) at 0-10°C , and stirred for 1 hour. A solution of tetrahydrofuran (180mL) containing ethyl tert-butoxycarbonyl-L-pyroglutamate (150g) was added dropwise at -15~-10°C, and reacted for 2h. After the reaction, add saturated ammonium chloride solution (700mL) and ethyl acetate (1000mL), separate the ethyl acetate, extract the aqueous phase with ethyl acetate (1000mL×3), combine the ethyl acetate phase, and add saturated ammonium chloride After washing (200 mL×1), drying over anhydrous sodium sulfate, the organic layer was concentrated to obtain compound II as a white solid (182 g, HPLC>99%, yield 89.3%).
[0049] [ 1 H NMR (CDCl 3 ),400MHz]δ: 1.29(3...
Embodiment 2
[0066] 1. Same as step 1 in Example 1.
[0067] 2. Synthesis of (S)-ethyl-2-[(tert-butoxycarbonyl)amino]-6-chloro-5-oxohexanoate (compound III')
[0068]
[0069]Add tetrahydrofuran (120 mL) solution, compound II (20 g), and lithium chloride (2.92 g) to the reaction flask in sequence. A solution of tetrahydrofuran (20 mL) containing methanesulfonic acid (6.57 g) was added dropwise at -10 to 0° C., and the mixture was incubated and stirred for 2 hours. React at a temperature of 20-35° C. for 16-20 hours. After the reaction was over, most of the solvent was removed under reduced pressure, ethyl acetate (40mL×3) and water (40mL) were added, the aqueous layer was removed, the organic layer was washed with saturated sodium chloride, dried over anhydrous sodium sulfate and filtered, the filtrate Concentration under reduced pressure gave an off-white solid (15 g, HPLC>94%, yield 84.8%) compound III', which was sealed and stored at low temperature. [ 1 HNMR (CDCl 3 ),400MHz]δ:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com