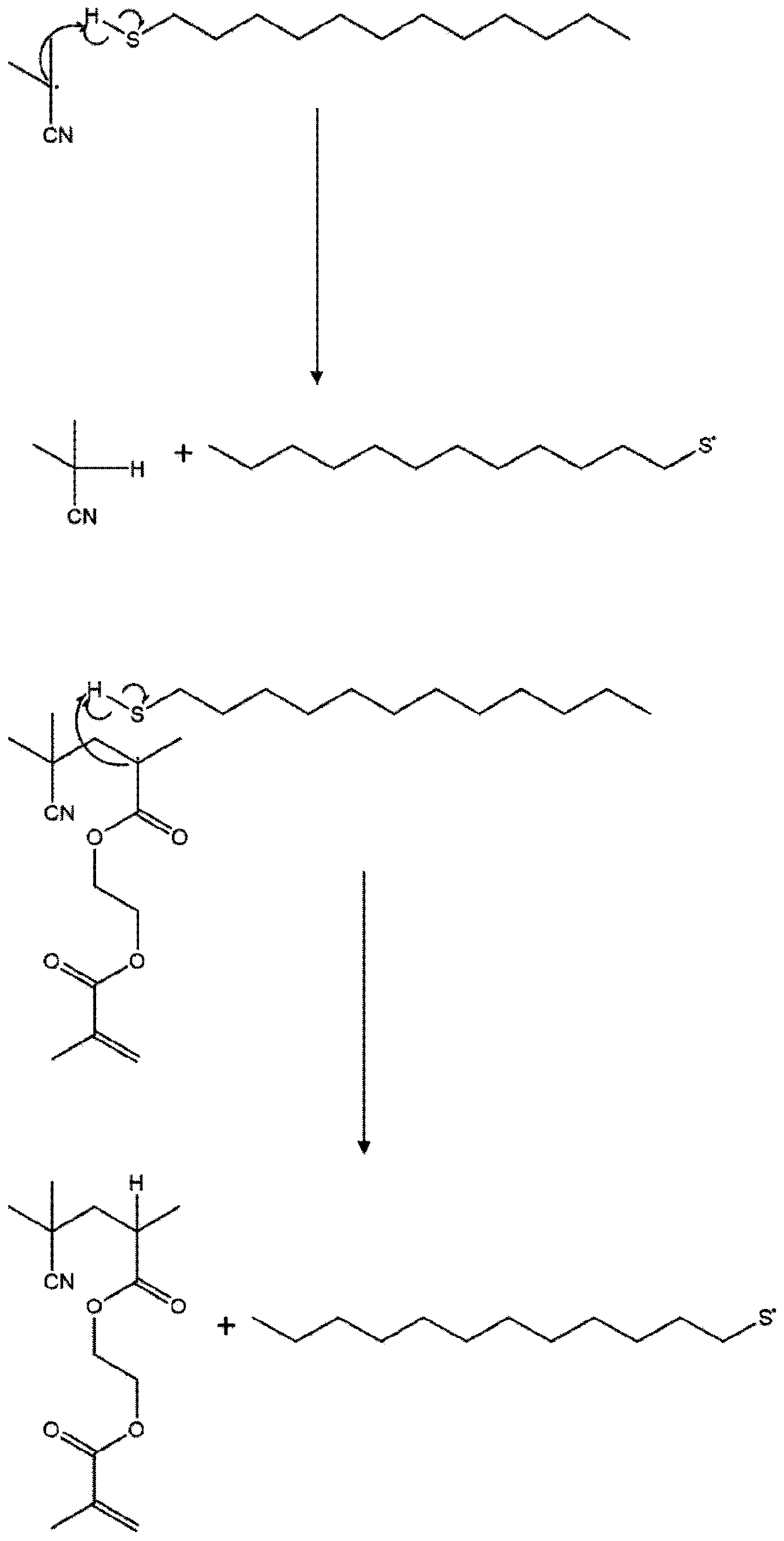
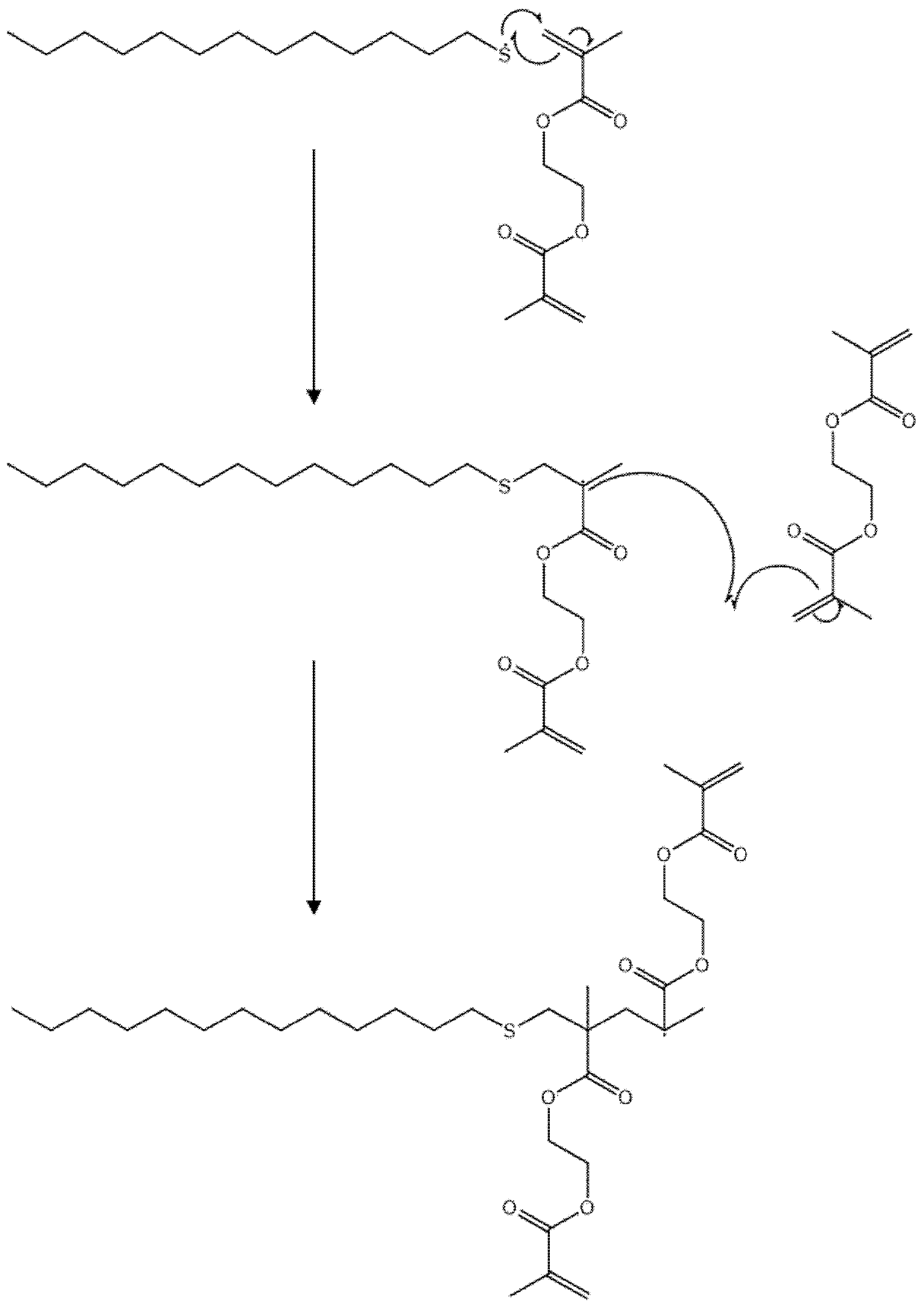
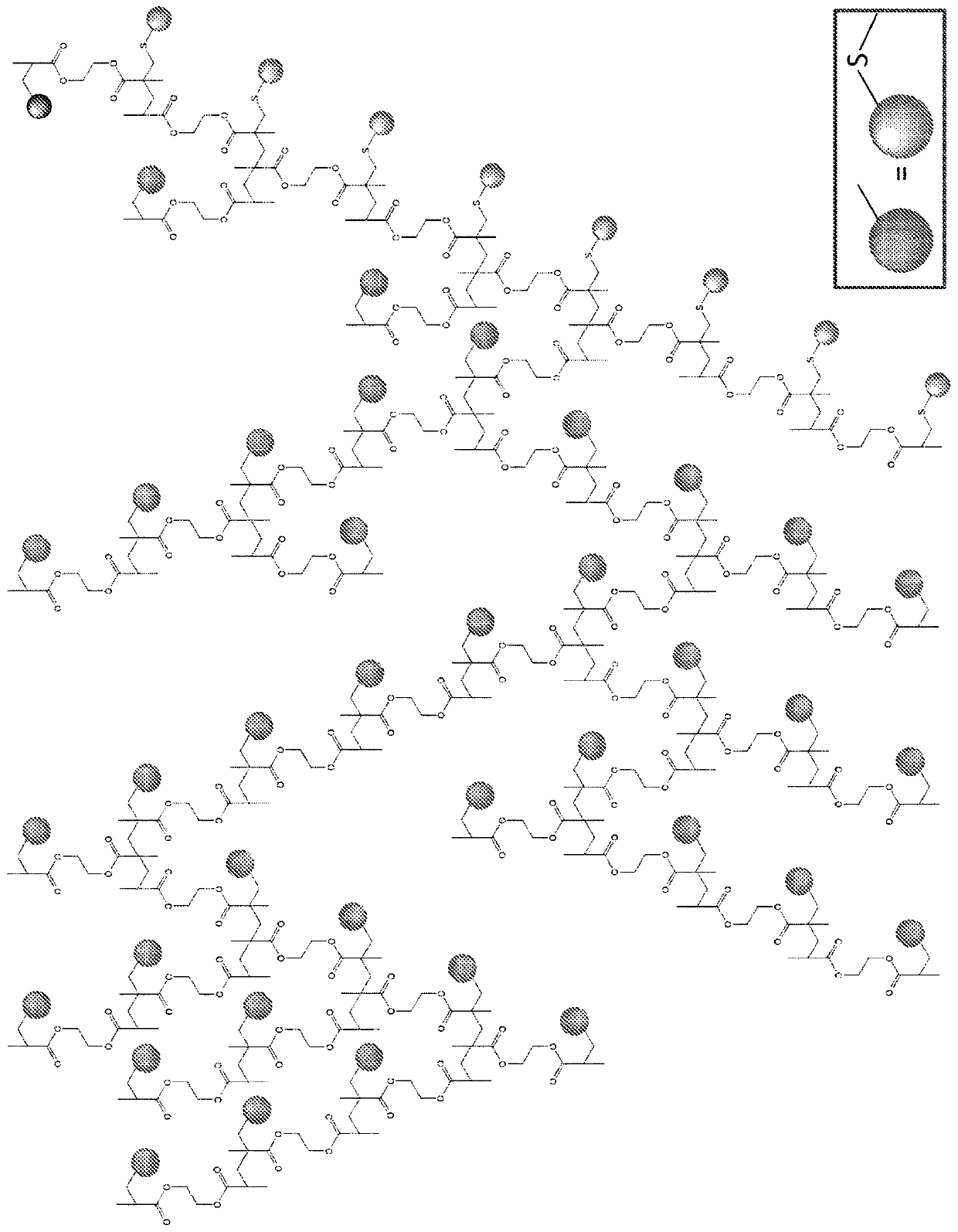
Polymers
A technology of polymers and polymer chains, applied in the field of branched polymers, to achieve an effect that is easy to control and does not require metal catalysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0244] Example 1 - EGDMA as divinyl monomer and DDT as chain transfer agent
[0245] Thus, in one embodiment, the divinyl monomer is EGDMA, the chain transfer agent is DDT, and a small amount of AIBN is used to provide a source of free radicals. The reaction can be carried out in toluene or other solvents.
[0246] Different ratios of chain transfer agent to divinyl monomer were investigated. A summary of the results is shown in the table below.
[0247] EGDMA – Monomer
[0248] DDT–CTA
[0249] AIBN – thermal initiator
[0250] Toluene – solvent (wt.50%)
[0251] Standard conditions:
[0252] · Oil bath at 70°C
[0253] Response time – 24 hours
[0254] The mass of AIBN is based on 1.5 mol% double bonds in the monomer
[0255]
[0256] a by CDCl 3 middle 1 H NMR (400MHz) measurement.
[0257] b As determined by triple detection GPC
[0258] c Scale up the reaction (3x the previous scale)
[0259] d Mark-Houwink parameter: [η]=KM a
[0260] e The reac...
Embodiment 2
[0265] Example 2 - EGDMA as divinyl monomer and benzyl mercaptan as chain transfer agent
[0266]
[0267] The details are the same as in Example 1, the difference is:
[0268] c The reaction took 72h
[0269] Purification by precipitation was performed using THF and ethanol at 0°C to give a white precipitate.
Embodiment 3
[0270] Example 3 - EGDMA as Divinyl Monomer and 2-Naphthalenethiol as Chain Transfer Agent
[0271]
[0272] The details are the same as in Example 1, the difference is:
[0273] a Cannot be analyzed as it is immiscible in the following solvent chosen: CDCl 3 , toluene and CDCl 3 , DMF and THF.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com