Preparation method of 6-substituted furyl-4-substituted aminoquinazoline derivatives and key intermediates thereof
A technology of aminoquinazoline and furanyl, applied in the field of medicinal chemistry, can solve the problems of poor stability of 2-formylfuran-5-boronic acid, unfavorable industrial production, poor stability of raw materials, etc., and achieve high yield and selectivity , low cost, less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
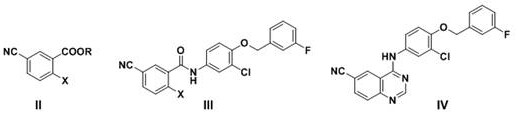
[0084] Example 1: Preparation of 6-cyano-4-[3-chloro-4-(3-fluorobenzyloxy)phenyl]aminoquinazoline (Ⅳ)
[0085] Step (1): Preparation of N-[3-chloro-4-(3-fluorobenzyloxy)phenyl]-2-bromo-5-cyanobenzamide (Ⅲ1)
[0086] In a 500 ml four-neck flask connected with stirring, a thermometer, and a reflux condenser, add 350 grams of toluene, 48.0 grams (0.2 moles) of methyl 2-bromo-5-cyanobenzoate (II1), 50.5 grams (0.2 moles ) 3-chloro-4-(3-fluorobenzyloxy)aniline, 1.5 g of ammonium chloride, stirred at 95 to 100° C. for 5 hours, and distilled off the produced methanol. Cool to 20-25°C, add 20 grams of water, separate layers, distill the organic phase to recover the solvent toluene, and recrystallize the residue with methyl tert-butyl ether to obtain 87.9 grams of N-[3-chloro-4-(3-fluorobenzyl Oxy)phenyl]-2-bromo-5-cyanobenzamide (Ⅲ1), yield 95.6%, liquid phase purity 99.8%.
[0087] Step (2): Preparation of 6-cyano-4-[3-chloro-4-(3-fluorobenzyloxy)phenyl]aminoquinazoline (IV)
[00...
Embodiment 2
[0090] Example 2: Preparation of 6-cyano-4-[3-chloro-4-(3-fluorobenzyloxy)phenyl]aminoquinazoline (Ⅳ)
[0091] Step (1): Preparation of N-[3-chloro-4-(3-fluorobenzyloxy)phenyl]-2-chloro-5-cyanobenzamide (Ⅲ2)
[0092] In a 500 ml four-neck flask connected with stirring, a thermometer, and a reflux condenser, add 350 grams of toluene, 42.0 grams (0.2 moles) of ethyl 2-chloro-5-cyanobenzoate (II2), 50.5 grams (0.2 moles ) 3-chloro-4-(3-fluorobenzyloxy)aniline, 2.0 g of zinc chloride, stirred and reacted at 95 to 100° C. for 5 hours, and distilled off the ethanol produced. Cool to 20-25°C, add 20 grams of water, separate layers, distill the organic phase to recover the solvent toluene, and recrystallize the residue with methyl tert-butyl ether to obtain 79.9 grams of N-[3-chloro-4-(3-fluorobenzyl Oxy)phenyl]-2-chloro-5-cyanobenzamide (Ⅲ2), yield 96.3%, liquid phase purity 99.7%.
[0093] Step (2): Preparation of 6-cyano-4-[3-chloro-4-(3-fluorobenzyloxy)phenyl]aminoquinazoline (I...
Embodiment 3
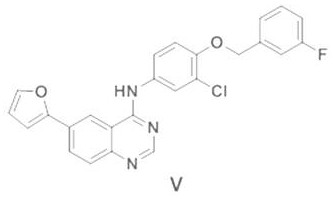
[0095] Embodiment 3: the preparation of lapatinib (program A)
[0096] Step (1): Preparation of 6-(furan-2-yl)-4-[3-chloro-4-(3-fluorobenzyloxy)phenyl]aminoquinazoline (Ⅴ)
[0097] Into a 500 ml four-neck flask connected with a stirring, thermometer, constant pressure dropping funnel and reflux condenser, add 100 g of tetrahydrofuran, 1.6 g of metal magnesium powder, 0.4 g of 1,2-dibromoethane, 1 millet grain size iodine, 30-45 ° C to initiate the reaction, and then between 40-45 ° C, dropwise add a mixed solution of 10.5 grams (0.06 moles) of 1,1-dimethoxy-3-bromopropane and 100 grams of tetrahydrofuran, about 2 The dropwise addition was completed within 1 hour, and then the reaction was stirred at 40-45°C for 2 hours. After cooling to 20-25°C, transfer the obtained Grignard reagent liquid to a constant pressure dropping funnel for use. In another 500 ml four-neck flask connected with stirring, thermometer and reflux condenser, 100 g of tetrahydrofuran, 20.2 g (0.05 mole) o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com