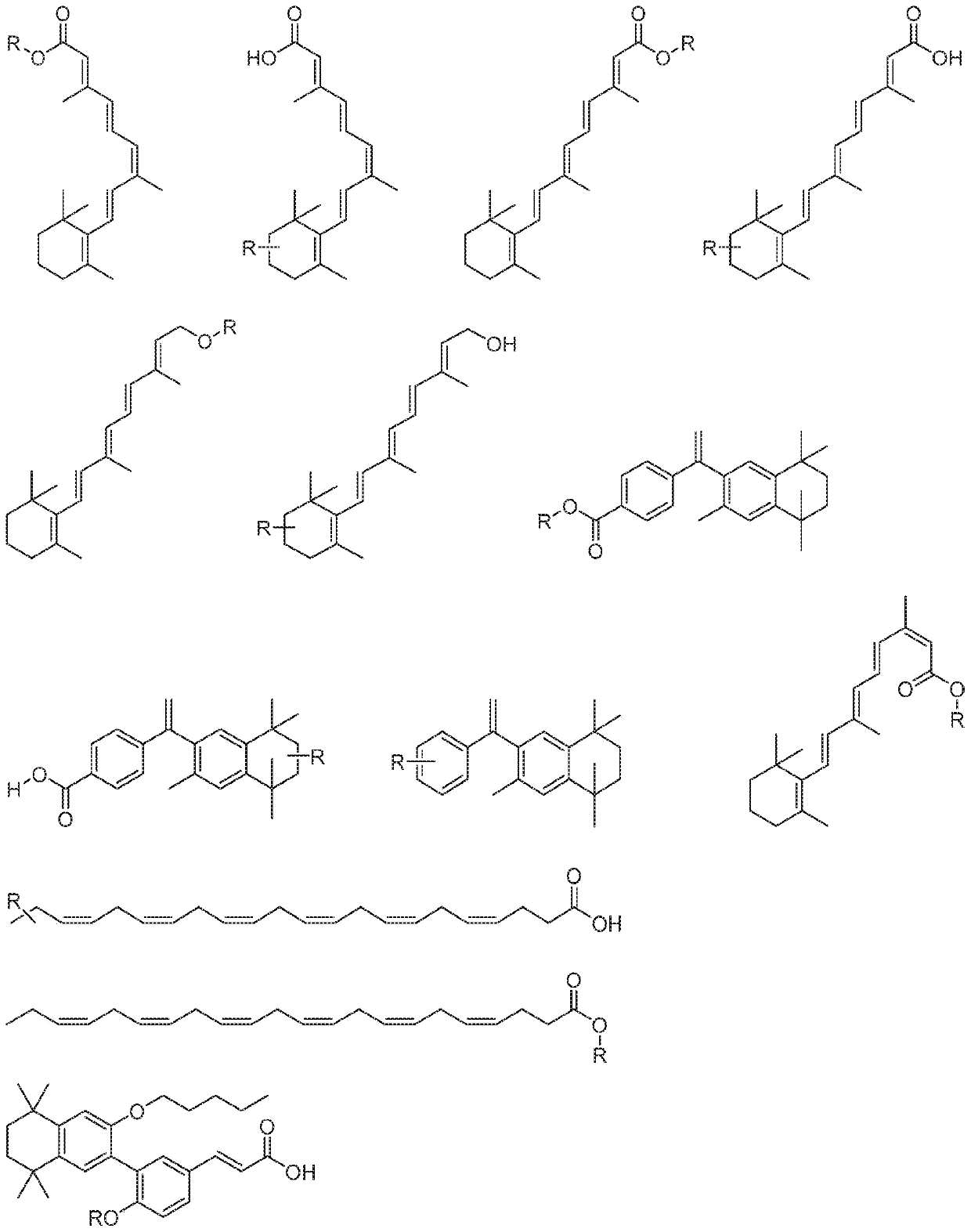
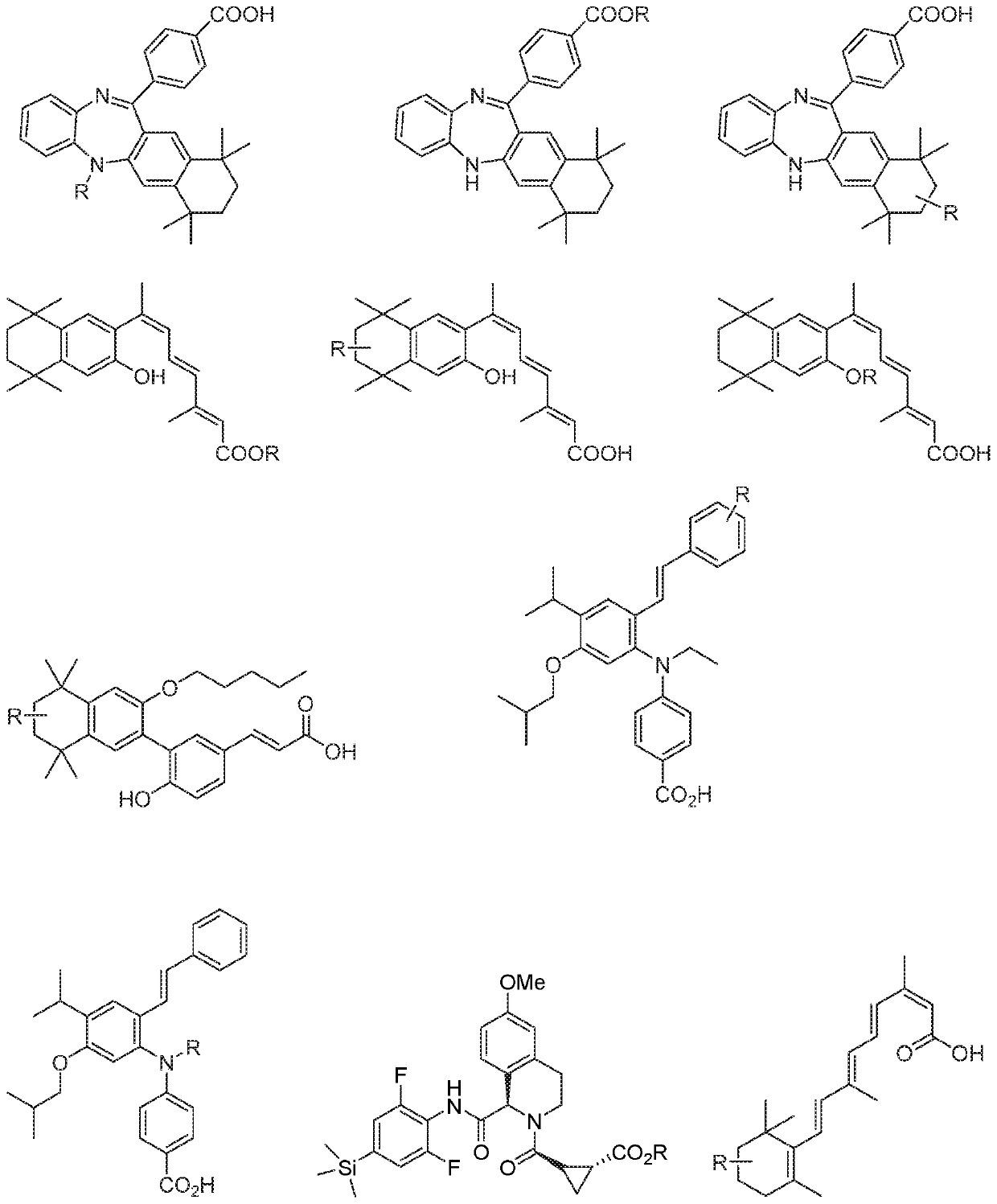
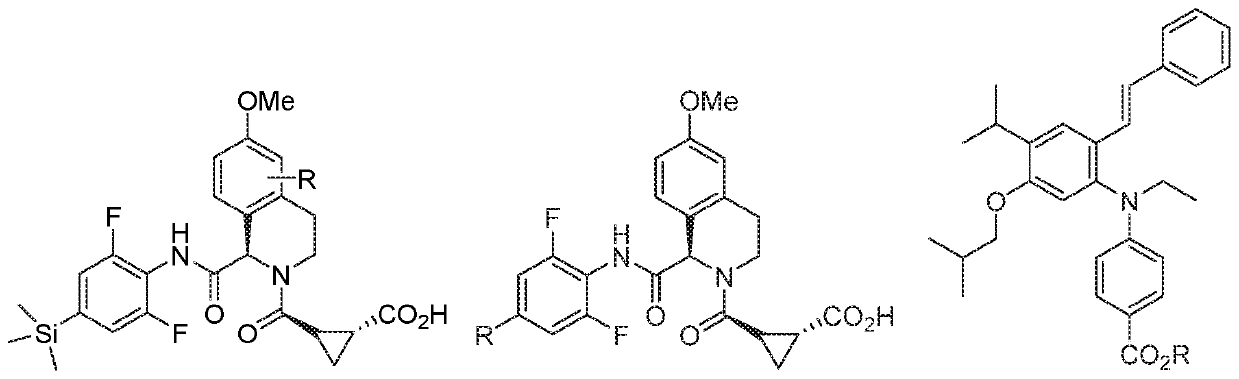
N/o-linked degrons and degronimers for protein degradation
A ligand and SO2 technology, applied in medical preparations containing active ingredients, organic chemistry, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0902] VII. Synthesis of Representative Compounds Example 1: General Synthesis of N-Linked Compounds of the Invention
[0903] Scenario 1: Common Procedures A and B
[0904]
[0905] General Procedure A
[0906] To a stirred solution of 1-2 (1.0 mmol) in DMF (3 mL) was added the aniline 1-1 (2.5 mmol). The resulting solution was heated at 80°C-100°C for 5-24 hours to yield 1-3. The reaction mixture was then cooled to room temperature and evaporated under reduced pressure. Following the procedure given below, the crude reaction material was purified by reverse phase preparative HPLC to give pure 1-3.
[0907] Non-limiting examples of compounds formed by General Procedure A include
[0908]
[0909] General procedure B
[0910] To a mixture of 1-1 (1 mmol) and 1-2 (2 mmol) in dioxane (3 mL) was added N,N-diisopropylethylamine (2 mmol). The resulting solution was heated at 70-110 °C for 24 hours in a sealed tube to yield 1-3. The reaction mixture was then cooled to r...
Embodiment 2
[1118] Example 2: N-heterocyclic anilino type
[1119] Scheme 7: Synthesis of compound 94:
[1120]
[1121] step 1:
[1122] Following the general procedure shown in Scheme 1, tert-butyl 4-(4-amino-pyrazol-1-yl)-piperidine-1-carboxylate compound 73 was synthesized using DIPEA / DMF. Yield -71.4%; LC MS: ES+378.3
[1123] Step 2:
[1124] To a pre-cooled solution of 4M dioxane-HCl was added compound 73 (46 mg, 121 μmol) at 0 °C, and the resulting mixture was stirred at ambient temperature for 3 hours to yield crude compound 94. The reaction mixture was concentrated under reduced pressure, and the resulting solid was triturated with ether-pentane to give Compound 94 (35.0 mg, 111 μmol, 92%) as a brown solid. 1 H NMR (400MHz, deuterium oxide) δ7.90(s, 1H), 7.68(s, 1H), 4.61(dt, J=12.0, 7.7Hz, 1H), 4.45(dd, J=13.2, 5.3Hz, 1H ), 3.63(d, J=13.0Hz, 2H), 3.25(t, J=13.1Hz, 2H), 2.86–2.77(m, 2H), 2.43–2.28(m, 2H), 2.31–2.17(m, 2H), 2.11 (dd, J = 12.2, 6.7 Hz, 1H); LC MS: ES+278....
Embodiment 3
[1134] Embodiment 3: the synthesis of N-alkyl compound of the present invention
[1135]
[1136] Following the general procedure shown in Scheme 1, compound 97 was synthesized using DIPEA / dioxane. Yield-19%; 1H NMR (400MHz, DMSO-d6) δ10.61(s, 1H), 4.58-4.52(m, 2H), 4.49-4.41(m, 2H), 4.27-4.19(m, 1H) , 3.54(dd, J=12.48, 4.6Hz, 1H), 2.60-2.55(m, 1H), 2.49-2.44(m, 1H), 2.29(s, 3H), 2.05-1.97(m, 1H), 1.78 -1.74 (m, 1H); LC MS: ES+199.3.
[1137]
[1138] Following the general procedure shown in Scheme 1, compound 98 was synthesized using DIPEA / dioxane. Yield - 22%; 1 H NMR (400MHz, DMSO-d6) δ10.62(s, 1H), 3.89-3.71(m, 5H), 3.62-3.56(m, 1H), 2.59-2.49(m, 2H), 2.21(s, 3H) ), 2.04-1.96 (m, 1H), 1.79-1.74 (m, 1H), 1.37 (s, 9H); LC MS: ES-296.28.
[1139]
[1140] Following the general procedure shown in Scheme 1, compound 99 was synthesized using DIPEA / dioxane. Yield-14%; 1H NMR (400MHz, DMSO-d6) δ10.73(s, 1H), 7.64-7.60(m, 2H), 7.36(t, J=7.76Hz, 2H), 7.12(t, J =7.12H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com