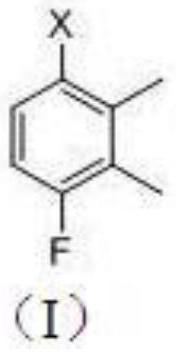
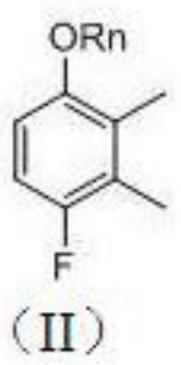
A kind of synthetic method of 4-fluoro-2,3-dimethylphenyl methanesulfonate
A technology of dimethylphenyl mesylate and dimethylphenyl ether, which is applied in the field of organic synthesis, can solve the problems of expensive palladium-carbon noble metal catalysts, increased preparation costs, and high environmental protection pressure, so as to reduce the difficulty of synthesis, The effect of reducing preparation cost and reducing environmental pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] This embodiment includes the following steps:
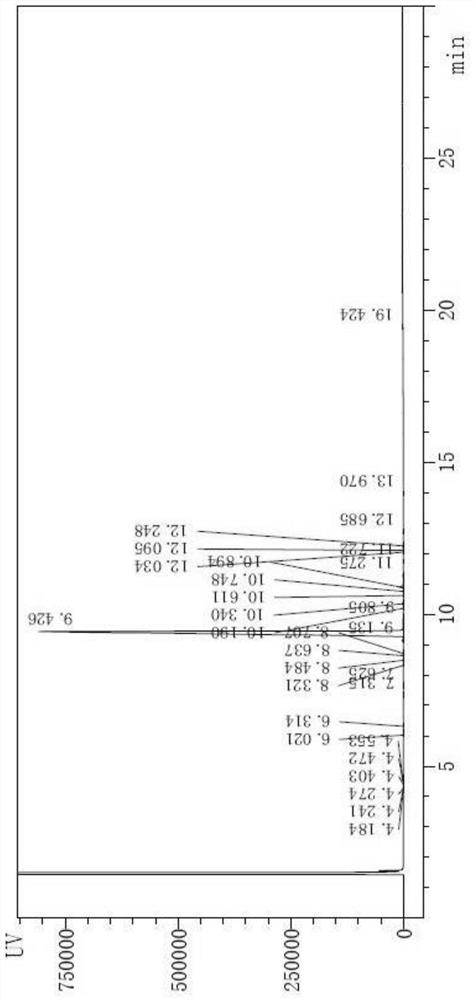
[0029] Step 1. Add 120g of acetic acid into a 500mL three-necked flask, then turn on the stirring device for stirring, add 30g of 3-fluoroxylene and 0.3g of iodic acid in turn, and then control the temperature in the three-necked flask to -5~5℃. 42g of bromine was added dropwise, and after the dropwise addition was completed, the reaction was maintained at -5 to 5°C for 8 hours to obtain a reaction solution containing 4-fluoro-2,3-dimethylbromobenzene; the solution containing 4-fluoro-2,3 -The reaction solution of dimethylbromobenzene is added to 2400g of water, and then 210g of DCM (methylene chloride) is added for extraction, and an equal amount of DCM is added to extract three times in total, and the organic phases separated after the three extractions are combined, Then add the sodium bicarbonate solution that 10g sodium bicarbonate is prepared with 1000g water, 1000g water wash successively, the organic phase that obt...
Embodiment 2
[0039] This embodiment includes the following steps:
[0040] Step 1. Add 240g of acetic acid into a 1000mL three-necked flask, then turn on the stirring device for stirring, add 60g of 3-fluoroxylene and 0.6g of iodic acid in turn, and then control the temperature in the three-necked flask to -5~5℃. 84g of bromine was added dropwise, and after the dropwise addition was completed, the reaction was maintained at -5 to 5°C for 8 hours to obtain a reaction solution containing 4-fluoro-2,3-dimethylbromobenzene; -The reaction solution of dimethylbromobenzene is added to 4800g water and then added 420g of DCM (dichloromethane) for extraction, each time an equal amount of DCM is added for a total of three extractions, the organic phases separated after three extractions are combined, Then add the sodium bicarbonate solution that 10g sodium bicarbonate is prepared with 1000g water, 1000g water wash successively, the organic phase that obtains is dried and filtered with 100g anhydrous ...
Embodiment 3
[0047] This embodiment includes the following steps:
[0048] Step 1. Add 240g of acetic acid into a 1000mL three-necked flask, then turn on the stirring device for stirring, add 60g of 3-fluoroxylene and 0.6g of iodic acid in turn, and then control the temperature in the three-necked flask to -5~5℃. 84g of bromine was added dropwise, and after the dropwise addition was completed, the reaction was maintained at -5 to 5°C for 8 hours to obtain a reaction solution containing 4-fluoro-2,3-dimethylbromobenzene; -The reaction solution of dimethylbromobenzene is added to 4800g water and then added 420g of DCM (dichloromethane) for extraction, each time an equal amount of DCM is added for a total of three extractions, the organic phases separated after three extractions are combined, Then add the sodium bicarbonate solution that 10g sodium bicarbonate is prepared with 1000g water, 1000g water wash successively, the organic phase that obtains is dried and filtered with 100g anhydrous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com